作者简介:柯丹霞(1983-),女,湖北十堰人,博士。
半胱氨酸蛋白酶抑制剂(cystatin)参与豆科植物与根瘤菌共生结瘤过程。克隆了大豆的1个半胱氨酸蛋白酶抑制剂基因 GmCYS20,基因编码区全长为291 bp。氨基酸多序列比对分析发现,大豆GmCYS20蛋白与野生大豆cystatin蛋白相似性高达99%。构建 GmCYS20基因的植物表达载体,通过发根农杆菌介导的百脉根发状根转化法获得转 GmCYS20基因复合体植株,利用GUS染色和RT-PCR方法鉴定阳性发状根。复合体植株接种根瘤菌4周后,转 GmCYS20基因植株结瘤数目明显高于空载体对照,结瘤指示基因的转录水平也呈上升趋势。进一步对接种10 d复合体植株中根瘤菌的侵染表型进行统计发现,过量表达 GmCYS20基因并不影响根瘤菌的侵染过程,而是在根瘤起始与发育阶段发挥正调控作用。结果表明: GmCYS20基因通过促进根瘤的起始与发育,从而参与调控共生结瘤过程。研究结果可为揭示豆科植物与根瘤菌的共生互作机制提供新的分子生物学证据。
Cystatin plays an important role in the symbiotic relationship between leguminous plants and rhizobium. We cloned the soybean cystatin gene GmCYS20 (291 bp) for analysis in this study. The analysis of amino acid multiple sequence alignment with homologous proteins showed that the similarity between soybean GmCYS20 and wild soybean ( Glycine soja) CYS was up to 99%. The plant overexpression vector was constructed and GmCYS20-overexpression composite plants were obtained in Lotus japonicas using the hairy root transformation method. Positive hairy roots were identified by GUS staining and RT-PCR assay. After inoculation with rhizobia for 4 weeks, overexpression of GmCYS20 increased the number of nodules in composite Lotus japonicus and up regulated the expression levels of symbiotic related genes. Further analysis of rhizobial infection phenotypes after inoculation with rhizobia for 10 days showed that overexpression of GmCYS20 gene did not affect the rhizobium infection process but played a positive role in initiation and development of nodules. These results showed that the GmCYS20 gene was involved in the regulation of symbiotic nodulation by promoting nodule initiation and development in L. japonicus. The results provide new molecular biological avenues to reveal the mechanisms of the symbiotic interaction between leguminous plants and rhizobium.
半胱氨酸蛋白酶抑制剂(cystatin)在生物体内广泛存在[1], 是一类可以与半胱氨酸蛋白酶特异性结合, 并抑制其蛋白水解酶活性的蛋白质[2]。Cystatin蛋白首先被报道参与调控植物发育过程, 包括严格控制植物体内蛋白质的降解[3], 参与贮藏蛋白的沉积和调运等生理过程[4, 5, 6]。此外, cystatin蛋白通过调控半胱氨酸蛋白酶的活性, 从而延缓叶片衰老过程。如康乃馨(Dianthus caryophyllus)cystatin 基因Dc-CPIn可以抑制花瓣的萎蔫[7]。水稻(Oryza sativa)cystatin 基因OC-I在烟草(Nicotiana tabacum)中的异源表达延长了烟草的营养生长阶段, 开花时间和叶片衰老过程被推迟[8]。苹果(Malus prunifolia)cystatin 基因MpCYS4也能够延缓叶片的衰老过程[9]。
近年来, 研究发现cystatin基因在提高植物对非生物胁迫的抗性方面具有重要作用[10, 11]。水稻cystatin 基因OC-I通过调控半胱氨酸蛋白酶活性影响植株对胁迫的抗性[12]。拟南芥(Arabidopsis thaliana)AtCYS4和AtCYS5基因受高温诱导表达, 基因的过表达增强拟南芥的耐高温能力[13, 14]。苹果MpCYS2[15]、MpCYS4[16]和MpCYS5[17]基因分别提高转基因拟南芥对干旱、脱落酸(abscisic acid, ABA)和盐胁迫的抗性。野生大豆(Glycine soja) cystatin蛋白GsCPI14通过与钙结合类受体蛋白激酶GsCBRLK相互作用, 参与碱胁迫反应[18]。另外, 植物cystatin蛋白对鳞翅目、鞘翅目等有害昆虫的生长抑制作用[19, 20]以及cystatin参与植物对病原菌入侵的防御过程[21, 22] 等方面也得到了较为深入的研究。
综上, 大量证据表明植物cystatin基因在调控植株发育, 提高植物对非生物胁迫和病虫害的抗性等方面具有重要作用。但关于该类基因在豆科植物共生结瘤过程中的功能研究报道较少。目前已经在大豆(Glycine max)中分离并鉴定了20个cystatin基因, 它们在大豆的14个不同组织中均有表达[23], 其中7个基因在根瘤中具有转录活性[24]。Yuan等[25]细致描述了20个大豆cystatin基因的全基因组特征, 并对该类基因在接种根瘤菌后不同时期的根及根瘤中的表达特征进行了检测。其中, GmCYS20基因在接种后0.5 h的根中表达量迅速升高, 在随后的7~24 h、5、16以及21 d根中表达量持续上升, 维持较高的转录水平。推测GmCYS20可能在大豆共生结瘤过程中发挥功能。为了明确该基因的生物学功能, 本研究在前人工作基础上克隆了大豆GmCYS20基因, 在豆科模式植物百脉根中异源表达该基因, 通过对复合体百脉根共生结瘤表型以及根瘤菌侵染表型的鉴定, 揭示GmCYS20基因在共生结瘤过程中的功能, 为进一步阐明该类基因在豆科植物共生结瘤过程中的分子机制奠定基础。
大豆测序品种Williams82(W82)种子由中国科学院生态与地理研究所孔凡江研究员提供; 百脉根(Lotus japonicus)MG-20种子、改造的植物表达载体p1302G(携带GUS标签)、百脉根根瘤菌PN28(携带LacZ标签的MAFF303099菌株)由华中农业大学农业微生物国家重点实验室张忠明教授提供。
W82大豆种子表面灭菌后, 种脐朝下平铺于无菌润湿滤纸上, 28 ℃暗培养待萌发。收集新鲜大豆根尖组织, 液氮速冻。参照RNA提取试剂盒(Invitrogen公司, USA)说明书, 提取大豆根尖组织总RNA。按照TIANGEN公司反转录试剂盒操作说明获得cDNA 第一链。根据https://www.soybase.org/search/网站公布的大豆GmCYS20基因(Glyma.20g045500)序列设计引物F-GmCYS20和R-GmCYS20(表1), PCR扩增GmCYS20目的基因。回收目的片段, 连接T载体, 送南京金斯瑞公司测序验证。设计引入酶切位点EcoR I和Sma I的引物F-OX和R-OX(表1), 将测序正确的目的基因插入植物表达载体p1302G(Gus基因作为筛选标记基因)中, 构建p1302G-GmCYS20重组质粒, 经冻融法转入发根农杆菌LBA1334中备用。
| 表1 本研究中所使用的引物 Table 1 The primers used in this study |
利用NCBI网站的Blastp工具寻找栽培大豆(Glycine max)GmCYS20蛋白(NP_001239817.1)的同源蛋白, 包括野生大豆(Glycine soja, KHN35444.1), 绿豆(Vigna radiata, XP_014516943.1), 木豆(Cajanus cajan, XP_020236689.1), 赤豆(Vigna angularis, XP_017411678.1), 鹰嘴豆(Cicer arietinum, XP_012574834.1), 蒺藜苜蓿(Medicago truncatula, XP_003599710.2), 狭叶羽扇豆(Lupinus angustifolius, XP_019419326.1), 紫花苜蓿(Medicago sativa, AAZ98791.1), 百脉根(AFK41117.10), 栽培花生(Arachis hypogaea, CBX19819.1), 蔓花生(Arachis duranensis, XP_020995318.1)和落花生(Arachis ipaensis, XP_016197069.1)共12种不同豆科植物的cystatin同源蛋白。利用DNAMAN软件进行同源蛋白的多序列比对和进化树分析。
首先对百脉根种子进行表面灭菌, 置于润湿滤纸上22 ℃黑暗培养, 待其两片子叶展开, 下胚轴长至1 cm长时, 剪去根部, 获得用于侵染的外植体。用携带有重组质粒p1302G-GmCYS20的LBA1334农杆菌菌悬液(OD600=0.6)浸泡外植体30 min, 置于MS培养基上, 22 ℃暗培养3~5 d。随后将外植体移入含300 mg· L-1头孢霉素的MS培养基上, 22 ℃培养2~3周, 待发状根长出后, 标记每一条发状根并剪下发状根根尖部分, 浸泡于GUS染液[26]中37 ℃过夜反应。观察根尖显色情况, 与平板上的发状根一一对应, 将不变蓝的阴性发状根彻底剪掉, 留下变蓝的阳性发状根继续培养。随机挑选部分阳性发状根, 进行RT-PCR检测。提取阳性发状根总RNA, 反转录后用表1中F-GmCYS20和R-GmCYS20引物扩增GmCYS20基因, 以百脉根多聚泛素(Polyubiquitin, UBI)为内参基因, 引物为F-UBI和R-UBI。
将上述复合体植株种入无菌沙盆, 炼苗2~3 d, 第4天接种根瘤菌PN28, 每天浇灌无氮营养液1次。接种4周后, 分别将超表达GmCYS20复合体植株(GmCYS20-OX)和空载体对照p1302G复合体植株从沙盆中取出, 用蒸馏水清洗根系, 对结瘤表型进行统计拍照。根据单株结瘤数和样本量(n), 计算单株平均结瘤数, 试验重复两次, 取平均值, 于2017年8月完成。
利用表1中GmCYS20和结瘤相关基因NIN、ENOD40-1和ENOD40-2的荧光定量引物检测复合体植株阳性发状根中各基因的转录水平, 百脉根UBI基因作为内参。按照Takara公司PrimeScript RT reagent Kit操作说明进行荧光定量PCR(Real-time PCR)检测, 根据相对定量法(2-△ △ Ct:用于比较不同样品之间的变化比率)公式计算结果。试验重复3次, 采用Excel 2007和SPSS 13.0软件进行数据分析, 并绘制图表。
同1.5结瘤试验, 复合体百脉根植株接种PN28根瘤菌10 d后, 取出植株, 将发状根清洗干净, 每棵植株剪下2~4条4~6 cm左右的发状根, 放入2%戊二醛溶液中固定2~3 h, 使内源β -gal失活, 然后用pH 7.4的PBS缓冲液清洗发状根2~3次, 去掉残留的戊二醛溶液。最后将发状根放入LacZ染液[27]中, 30 ℃染色过夜。将染色处理后的发状根转移到PBS缓冲液中, 制作水浸片, 光学显微镜下观察发状根中根瘤菌的侵染情况。按照根瘤菌吸附到根毛顶端、侵入线进入根表皮、根瘤菌进入根瘤原基和成熟根瘤形成这4个阶段统计复合体植株发状根中根瘤菌的侵染表型。试验于2017年9月完成。
根据大豆GmCYS20基因的已知序列设计引物, 以大豆根尖组织cDNA为模板, PCR扩增目的条带。经测序验证, 克隆片段长度为291 bp, 碱基数及序列与网站公布序列完全一致, 说明已经成功克隆了GmCYS20基因(图1A), 该基因在大豆中的ID号是Glyma.20G045500, 位于大豆基因组20号染色体的8475315~8478532位, 含有2个外显子和1个内含子。序列分析表明(图1B), GmCYS20基因的CDS区编码97个氨基酸残基, 蛋白质的等电点(PI)为5.83, N端无信号肽, 三维结构包含2个α 螺旋和6个β 折叠。GmCYS20属于第Ⅲ 类cystatin蛋白, 含有该家族共有的4个保守结构域GG、LARFAVE、QVVSG和SW。
在NCBI网站上对GmCYS20基因的编码序列进行Blastp比对, 寻找其他豆科植物中GmCYS20的同源蛋白。结果发现, GmCYS20与野生大豆、绿豆、木豆、赤豆、鹰嘴豆、 蒺藜苜蓿、狭叶羽扇豆、紫花苜蓿、百脉根、栽培花生、蔓花生和落花生等12种豆科植物的CYS蛋白相似性较高(图2)。通过DNAMAN软件获得大豆GmCYS20蛋白与上述12种豆科植物CYS蛋白的进化树, 由图3可知, 大豆GmCYS20蛋白与野生大豆CYS蛋白处在同一进化分支上, 亲缘关系最近。
 | 图2 大豆GmCYS20与其他豆科植物同源蛋白的多序列比对Fig.2 Multiple sequence alignment of GmCYS20 with its homologs in other leguminous plants |
为了明确GmCYS20基因在共生结瘤过程中的生物学功能, 在模式豆科植物百脉根中对GmCYS20基因进行超量表达的研究。将克隆的GmCYS20基因插入改造过的植物表达载体p1302G中, 重组质粒经EcoR I和Sma I双酶切后获得大小正确的载体片段和目的片段(图4A), 将质粒送交公司测序。利用冻融法将测序结果正确的质粒转入发根农杆菌LBA1334, 鉴定后保存于-80 ℃, 用于百脉根的遗传转化。
参照1.4中的百脉根发状根转化流程, 制备转GmCYS20基因的复合体百脉根。首先对切口处新长出的发状根进行GUS染色。标记每一条发状根并剪下其根尖部分, 浸泡于GUS染液中37 ℃过夜反应。结果如图4B所示, 变蓝的为阳性发状根, 箭头所指为不变色的阴性发状根。根据标记, 将平板上对应的阴性发状根彻底剪掉, 留下阳性发状根进行盆栽试验。复合体百脉根接种根瘤菌4周后, 随机挑选部分发状根进行GUS染色, 结果发现发状根均呈蓝色(图4C)。与此同时, 随机挑选部分发状根, 进行RT-PCR检测。结果显示, 检测的转GmCYS20基因发状根中均出现较强的转录信号, 而空载体对照中没有出现目的条带(图5), 说明外源基因GmCYS20已经成功整合到GmCYS20-OX百脉根发状根基因组中。
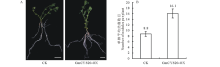
对上述鉴定为阳性的复合体百脉根植株进行结瘤试验。接种根瘤菌4周后, 统计GmCYS20-OX组和空载体对照组的结瘤表型发现, GmCYS20-OX组的单株平均结瘤数明显高于对照组。GmCYS20-OX组的单株平均结瘤数为8.8个, 而空载体对照组的单株平均结瘤数为16.1个(图6)。说明GmCYS20基因在百脉根中的异源表达, 能够显著增加百脉根根瘤数目, GmCYS20基因在百脉根的结瘤过程中起着积极的促进作用。
利用Real-time PCR方法检测转基因发状根中GmCYS20基因的表达, 结果显示GmCYS20基因在GmCYS20-OX发状根中的表达水平为空载体对照(CK)的5.5倍(图7), 说明植物超表达载体的构建有效, GmCYS20基因在GmCYS20-OX发状根中确实是过量表达的。接着检测结瘤指示基因NIN、ENOD40-1和ENOD40-2在转基因发状根中的转录水平, 进一步分析GmCYS20基因的过量表达对结瘤指示基因表达水平的影响。结瘤起始基因NIN是侵入线和根瘤原基形成的关键基因, 结瘤素基因ENOD40-1和ENOD40-2是根瘤起始和发育的关键基因[28]。由图7可见, 3个结瘤指示基因在GmCYS20-OX发状根中的表达水平均显著增加, 特别是ENOD40-2基因, 为对照的6.2倍。结果表明GmCYS20基因的过量表达上调结瘤指示基因的表达。
为了进一步明确GmCYS20基因在根瘤形成的哪个阶段发挥功能, 对接种根瘤菌10 d的转基因发状根进行染色处理, 光学显微镜下观察发状根中根瘤菌侵染宿主植物及根瘤的形成过程。按照根瘤菌吸附到根毛顶端、侵入线进入根表皮、根瘤菌进入根瘤原基和成熟根瘤形成这4个阶段统计复合体植株发状根中根瘤菌的侵染表型(图8A~D)。结果表明GmCYS20-OX复合体植株在根瘤菌进入根瘤原基以及成熟根瘤形成这两个阶段的统计数目较对照明显增多, 而在根瘤菌吸附到根毛顶端和侵入线进入根表皮这两个阶段与对照组并无明显差异(表2)。说明GmCYS20-OX复合体植株根瘤数目的增多是由根瘤原基增多导致的, 与根瘤菌的侵染无关。以上数据证实GmCYS20基因与根瘤的起始和发育相关, 是一个重要的共生信号调节因子。
| 表2 根瘤菌侵染表型的数据统计 Table 2 Data statistics of the rhizobial infection phenotype |
百脉根是一种优良的豆科牧草, 其固氮作用能够增加土壤肥力, 因此被广泛应用于补播改良草场、建立人工放牧地和人工草地。同时, 百脉根的细胞再生性能好, 遗传转化效率高, 目前已经成为实验室研究生物固氮机理、外源基因转化和牧草品质改良的模式豆科植物。Hansen等[29]利用发根农杆菌(Agrobacterium rhizogenes)最早建立了“ 复合体植株” 系统, 此系统主要由非转化的地上部分和转化的发状根组成。发根农杆菌介导的百脉根发状根转化系统, 具有遗传操作简便、转化效率高和稳定性好等突出优点。每个转基因发状根代表一个独立的转化事件[30]。该系统目前已经被广泛应用于生物固氮领域基因功能的研究[31, 32, 33, 34]。由于大豆遗传转化效率相对较低, 研究候选基因功能费时耗力[35], 因此本研究利用发根农杆菌介导的百脉根发状根转化系统来验证大豆候选基因GmCYS20在共生结瘤途径中的生物学功能, 快速高效评价该候选基因在目标作物— — 大豆中的育种利用价值。
半胱氨酸蛋白酶数量众多且广泛存在于豆科植物中, 几乎参与植物生长发育的各个方面, 包括萌发、昼夜节律、植物衰老和程序性细胞死亡等, 在根瘤发育、胁迫调节以及根瘤衰老控制过程中也有许多半胱氨酸蛋白酶参与其中[36]。研究表明, 基因沉默紫云英(Astragalus sinicus)半胱氨酸蛋白酶基因AsNODF32, 根瘤发育和类菌体衰老被延缓, 根瘤寿命被延长[37]。抑制蒺藜苜蓿半胱氨酸蛋白酶CYP15A也可以延缓根瘤的衰老[38]。苜蓿根瘤衰老的过程中伴随着一个保守的半胱氨酸蛋白酶亚家族MtCP1~MtCP6的表达, 此亚家族蛋白可能涉及共生体结构的降解[39], 这些半胱氨酸蛋白酶的表达或活性受抑制因子的调控。豌豆半胱氨酸蛋白酶PsCyp15A基因的表达在根瘤开始衰老时被激活[40]。
1983年最早报道了大豆中18个半胱氨酸蛋白酶在根瘤发育和衰老过程中维持较高的转录水平[41] 。综合最新的大豆共生相关数字基因表达谱[42, 43]以及根瘤样品转录组数据[24]发现, 有28个Papain-like半胱氨酸蛋白酶可能参与结瘤、根瘤的发育及衰老, 但是否存在特异的半胱氨酸蛋白酶参与调控根瘤的发育目前并不明确。半胱氨酸蛋白酶抑制蛋白是半胱氨酸蛋白酶的天然抑制剂, 明确其在根瘤发育中的作用, 可有效揭示其调控的半胱氨酸蛋白酶参与根瘤发育的机理。本研究中获得的大豆cystatin蛋白GmCYS20可能也是通过调控特异的半胱氨酸蛋白酶活性, 从而参与根瘤的起始和发育过程。因此, 进一步点对点寻找GmCYS20蛋白对应的半胱氨酸蛋白酶底物, 验证二者之间的相互作用及酶活调节机制对于阐明cystatin蛋白在共生结瘤过程中的分子调控机理具有重要意义。
本研究克隆了大豆的1个半胱氨酸蛋白酶抑制剂基因GmCYS20, 分析发现GmCYS20蛋白是一个典型的半胱氨酸蛋白酶抑制剂。通过对转GmCYS20基因复合体百脉根植株的共生表型鉴定发现, GmCYS20基因正调控根瘤的起始与发育。研究结果揭示了GmCYS20基因在共生结瘤过程中的生物学功能, 为进一步阐明该类基因在豆科植物共生结瘤过程中的分子机制奠定了理论基础, 同时, 也为揭示豆科植物与根瘤菌的共生互作机制提供了新的分子生物学证据。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|