作者简介:段慧荣(1987-),女,山西长治人,助理研究员,博士。E-mail: duanhuirong@caas.cn
K+是植物生长必需的营养元素。K+被植物根系吸收后,有效地向地上部转运,在跨膜转运的过程中主要由次级K+转运蛋白和K+通道介导。KT/HAK/KUP和HKT家族是参与植物体内K+吸收及转运的两类主要K+转运蛋白,其中HKT家族参与K+转运的成员仅存在于单子叶植物中,它们在植物生长发育、渗透调节等过程中均发挥重要作用。Shaker家族是K+通道中最早发现且研究最为深入的一类电压门控型通道,是植物K+吸收的重要途径之一。本研究从结构特征、定位和组织表达、功能调控等方面对植物KT/HAK/KUP家族、HKT家族和Shaker通道进行综述,最后对未来的主要研究方向做了展望。
As one of the major nutrients, potassium (K+) is essential for plant growth and development. Large amounts of K+ need to be taken up from the soil and transported throughout the plant. The K+ transporters and the K+ channels mainly mediate K+ transport across the plasma membrane and the tonoplast membrane in plants. The HAK/KUP/KT family and the HKT family are two main groups of proteins which have been widely associated with K+ uptake and transport, and they fulfill diverse roles in plant growth processes, salt tolerance and osmotic potential regulation. K+-permeable members of HKT family seem to be present in monocots only. The Shaker family, comprising voltage-gated channels, dominate the plasma membrane conductance of K+ in most environmental conditions. This review summarizes recent research and progress in understanding of the structure, localization, expression, function and regulation of three main K+ transport families. We also propose research hotspots and directions for future K+ research.
K+是植物中含量最多的阳离子, 也是植物生长必需的营养元素。作为重要的无机渗透调节剂和酶促剂, K+参与植物细胞内诸多生理及代谢过程。土壤盐碱化是限制作物生长、影响生态环境的一个主要非生物因素。植物体内积累过多的Na+会限制K+的吸收和转运, 扰乱植物对K+和其他矿质营养元素的吸收, 引起生长受抑甚至死亡。近年来, 有关植物体内K+吸收及转运的分子机制受到学术界的广泛关注。
K+被植物根系吸收后有效向地上部转运, 转运过程需通过质膜和液泡膜, 主要由次级K+转运蛋白和K+通道介导。参与植物体内K+吸收及转运的蛋白或通道共有5类:KT/HAK/KUP家族转运蛋白(KT/HAK/KUP K+ transporters)、HKT家族转运蛋白(HKT transporters)、CPAs(cation-proton antiporters)家族转运蛋白、Shaker通道(Shaker-like K+ channels)和TPK通道(tandem-pore K+ channels)[1]。早期, Epstein等[2]发现植物体内存在两条不同的K+吸收机制:机制Ⅰ 主要由K+转运蛋白介导外界低浓度(μ mol)下K+(Rb+)的吸收, 机制Ⅱ 主要由K+通道介导外界较高浓度(mmol)下K+(Rb+)的吸收。Coskun等[3]对拟南芥(Arabidopsis thaliana)和大麦(Hordeum vulgare)根中K+转运的两种机制重新进行评估后发现, 外界高浓度K+条件下, 阻断质外体途径的K+内流后, 低亲和性K+转运和高亲和性K+转运过程可同时存在, 这表明植物K+内流的适度上限受质膜转运蛋白控制。本研究对3类主要的K+转运蛋白或通道进行归纳总结, 从结构特征、定位和组织表达、功能调控等方面进行综合概述, 以期为今后深入研究植物K+的吸收及转运奠定基础。
KT/HAK/KUP属于APC(amino acid-polyamine-organocation)超级家族, 广泛存在于原核生物、真菌和高等植物中, 在低等植物如小立碗藓(Physcomitrella patens)和蕨(Selaginella moellendorffii)中也普遍存在[4]。该家族中的基因被不同研究者以不同的缩写命名, 包括KT, HAK和KUP。植物中最早克隆到的KT/HAK/KUP成员是大麦HvHAK1[5]和拟南芥AtKUP1/KT1, AtKUP2/KT2[6]。之后, 从玉米、甜椒(Capsicum annuum)、冰叶日中花(Mesembryanthemum crystallinum)、葡萄和番茄等植物中陆续克隆到该家族成员基因[7]。拟南芥、水稻、玉米和杨树中分别含有13、27、27和31个该家族成员(表1)[7]。
| 表1 KT/HAK/KUP、HKT和Shaker家族的比较 Table 1 Comparison of KT/HAK/KUP, HKT and Shaker family |
目前, KT/HAK/KUP家族晶体结构的研究尚不清楚, 该家族虽不具有完全相同的功能保守域, 但氨基酸序列中均含有GVVYGDLGTSPLY(加粗字母表示在所有基因中均保守), 疏水结构分析预测表明, 该家族蛋白成员含有10~14个跨膜区域(TM10-TM14)(表1)[8]。Nieves-Cordones等[9]从46个被子植物的全基因组测序结果中选出了913个KT/HAK/KUP家族成员, 通过系统进化分析, 可将该家族划分为5个亚族。亚族Ⅰ 成员以拟南芥AtHAK5、水稻OsHAK5和葡萄VvKUP1等为代表; 亚族Ⅱ 成员以拟南芥AtKUP1、AtKUP2、AtKUP3、AtKUP6、AtKUP8等为代表; 亚族Ⅲ 成员以AtKUP9、AtKUP10和AtKUP11等为代表; 亚族Ⅳ 成员以水稻OsHAK4和OsHAK17为代表; 亚族Ⅴ 成员以AtKUP5、AtKUP7和AtKUP12等为代表。亚族Ⅱ 和亚族Ⅲ 成员在双子叶和单子叶植物中的分布都很保守, 亚族Ⅰ 和Ⅳ 的成员则可能在植物适应环境中发挥特殊的作用, 这在双子叶植物拟南芥、葡萄、蒺藜苜蓿和单子叶植物水稻、玉米、短柄草中都可以得到验证[4]。
KT/HAK/KUP家族成员定位在植物不同细胞器的膜上, 如质膜、液泡膜、类囊体膜等[7, 10]。水稻OsHAK21定位在质膜上, 在木质部薄壁细胞和内皮层细胞中表达[11]。用融合绿色荧光蛋白(green fluorescent protein, GFP)方法将水稻OsHAK10瞬时表达在洋葱(Allium cepa)表皮细胞中, 发现它定位在液泡膜上[12]。拟南芥叶绿体蛋白质组中发现了AtKUP12的存在, 其定位在叶绿体膜上[13]。同一亚族的家族成员具有并不相同的细胞定位, 可能发挥着不同的细胞生物学功能。如亚族Ⅱ 成员拟南芥AtKUP4定位于液泡膜, 水稻OsHAK2和OsHAK3定位于细胞质膜, 陆地棉GhKT2定位在质膜上[14], 而OsHAK10则定位于液泡膜。
KT/HAK/KUP家族成员在大多数植物组织中均有表达, 在不同的组织中发挥各自不同的调节作用(表2)。正常生长条件下, 亚族Ⅰ 成员如大麦HvHAK1、水稻OsHAK1和OsHAK5、拟南芥AtHAK5、甜椒CaHAK1、 番茄LeHAK5、 梨(Pyrus bretschneideri)PbrHAK12/16和盐芥(Thellungiella halophila)ThHAK5在植物根和地上部中的表达量较低, 当遭受K+饥饿后, 表达水平显著上调[15, 16]。进一步研究发现, 这些成员的表达也受到其他离子如Na+和N
| 表2 不同高等植物中的KT/HAK/KUP家族成员 Table 2 Members of KT/HAK/KUP family in different plants |
KT/HAK/KUP转运蛋白在爪蟾卵母细胞(Xenopus oocytes)中不表达, 因此, 该家族成员的转运特性常用酵母(Saccharomyces cerevisiae)或大肠杆菌(Escherichia coli)等异源表达系统来验证[29]。KT/HAK/KUP家族成员能够恢复酵母或细菌K+吸收缺陷突变体的K+吸收能力, 在植物如拟南芥中也已经证明其在维持根和地上部的K+稳态平衡过程中起重要作用[21, 30]。该家族成员除能够转运K+外, 还能转运Rb+、Cs+等, 受N
亚族Ⅰ 成员在该家族中研究最广泛最深入, 已经被证明能参与双子叶和单子叶植物的高亲和性K+吸收, 其主要代表为拟南芥AtHAK5、大麦HvHAK1和水稻OsHAK1, 它们能够促进植物根系从外界低浓度K+环境中吸收K+, 从而适应低K+条件下的生长[13, 31, 32, 33]。亚族Ⅱ 成员根据转运特性和生理作用的不同具有功能多样性, 主要代表成员为拟南芥AtKT1/AtKUP1, AtKT2/AtKUP2, AtKT3/AtKUP4/TRH1和大麦HvHAK2。对单子叶植物而言, 亚族Ⅱ 的很多成员经常参与低亲和性K+吸收过程, 双子叶植物中此类成员表现出不同的转运活性, 如拟南芥AtKUP6介导低亲和性K+吸收[34], AtKUP4/TRH1介导高亲和性K+吸收[35]。AtKUP1介导兼性K+吸收, 在K+浓度100~200 μ mol· L-1时从高亲和性转为低亲和性K+吸收[36]。此外, Na+对该家族成员也有不同的影响。部分成员如HvHAK1, PhaHAK2和PhaHAK5可在mmol· L-1浓度范围内参与Na+的转运[5, 18]。抗盐芦苇品种的PhaHAK2-n和PhaHAK2-e均受K+饥饿和高盐胁迫的诱导, 可能在盐胁迫下持续发挥K+吸收功能, 从而维持体内高K+/Na+比, 提高芦苇耐盐性[37]。对AtHAK5或OsHAK1而言, Na+对其介导的K+吸收有明显的竞争抑制作用[31]。此外, 虽然OsHAK5与AtHAK5或OsHAK1的同源性很近, 但OsHAK5能够介导对Na+不敏感的K+吸收[38]。有关亚族Ⅲ 和Ⅳ 成员的研究报道较少。亚族Ⅲ 的成员AtHAK11可以介导K+和Rb+的吸收。盐敏感芦苇品种的PhaHAK5-u属于亚族Ⅳ 成员, 在酵母中被证实是高亲和性K+转运体, 在Na+存在时也参与介导低亲和性Na+转运[18]。亚族Ⅴ 的成员AtKUP7和AtKUP12略有研究, 其中AtKUP7参与K+亏缺条件下K+的吸收和转运过程, AtKUP12可能参与拟南芥的光合作用过程[39]。
在拟南芥中, 通过测定野生型和突变体(akt1或athak5)根中Rb+(或K+)动力学特征, 发现AtHAK5是外界K+浓度低于10 μ mol· L-1下介导K+吸收的唯一系统[40]。此外, 研究发现, 由于AtHAK5对Cs+的可渗透性, 在外界低K+条件下, AtHAK5能介导Cs+进入植物根中引起毒性反应[41]。亚族Ⅱ 转运蛋白能够影响植物发展过程, 尤其是细胞伸长过程:拟南芥AtKUP4/TRH1在根部和冠部都有大量表达, 其T-DNA插入突变体trh1的根毛伸长明显减缓, 参与植物激素的根特异分布, 影响根尖激素转运蛋白的极性定位, 能建立向地生长和根毛形成的激素梯度[35]。缺失AtKUP2/SHY3的突变体shy3会引起黑暗处理下地上部生长的缺陷, 表明AtKUP2能够影响细胞的生长, 但具体机制尚不清楚[30]。拟南芥atkup2/6/8突变体的生长受到促进, 植株的K+吸收特征结果表明, AtKUP2、AtKUP6和AtKUP8可能介导K+外流, 此外, 该突变体的保卫细胞和侧根细胞中对ABA敏感性的响应降低, 表明KT/HAK/KUP家族成员在植物侧根细胞对激素的敏感性响应过程中有重要作用[22]。普遍认为, 磷酸化是激活植物KT/HAK/KUP家族转运体活性的主要方式。Osakabe等[22]的研究发现, AtKUP6能被OST1磷酸化激活, 这表明AtKUP6是参与ABA介导的气孔关闭的调控因子。AtHAK5及其在其他物种中的同源物可被CIPK23/CBL1复合物激活, 捕蝇草(Dionaea muscipula)中也发现了DmHAK5类似的受CIPK23/CBL9复合物激活的现象[42]。
HKT高亲和性K+转运蛋白是广泛存在于真核生物和原核生物中的一类超级蛋白家族, 其中, 植物HKT转运蛋白与真菌和原核生物中Trk/Ktr阳离子转运蛋白家族的同源性很高[50]。Schachtman等[51]从小麦中得到的TaHKT2; 1, 是高等植物中的第一个高亲和性K+转运蛋白, 后被证实是K+-Na+共转运蛋白。随后, 研究者相继从不同植物如拟南芥、赤桉(Eucalyptus camaldulensis)、水稻和冰叶日中花等中克隆并鉴定了其同源基因。根据异源表达系统中对Na+、K+转运特性的不同, HKT可被分为两类:第Ⅰ 类以AtHKT1; 1为代表, 主要转运Na+, 如HvHKT1; 1/2, OsHKT1; 2和TaHKT1; 1/2等; 第Ⅱ 类以TaHKT2; 1为代表, 功能是K+∶ Na+共转运, 包括EcHKT1; 1/2, HvHKT2; 1, OsHKT2; 1, OsHKT2; 2等, 双子叶植物中缺少第Ⅱ 类基因成员[52]。
Durell等[53]认为植物HKT蛋白含有4个MPM高度保守结构和1个甘氨酸-酪氨酸-甘氨酸(GYG)基序, 是由仅有一个MPM结构的原核生物KcsA类K+通道经复制加倍及融合进化而来的。按照此模型, 植物HKT类转运体由8个跨膜结构域、4个P环、短的N-末端和C-末端组成, 其中4个MPM结构(1个MPM结构包括2个跨膜螺旋和1个P环)形成四倍径向对称结构, 4个P环与中央的疏水结构形成蛋白的渗透途径, 为该蛋白最狭窄的区域, 作为选择性过滤器(表1)。HKT转运蛋白的结构模型最初是通过序列分析得到的, 后在生化层面得到证实, 最近的原核生物Trk/Ktr类转运蛋白的晶体结构研究为此提供了进一步的证据[54]。此外, HKT蛋白在P环或其附近有多个K+∶ Na+选择性吸收位点。HKT的第一个P环过滤器位置处有1个氨基酸残基对其离子转运特性起着调控作用:第Ⅰ 类转运蛋白通常第1个P环中过滤器处为丝氨酸残基(S), 第Ⅱ 类转运蛋白在相应位置处常为甘氨酸残基(G)[55]。进一步研究发现, 介导K+∶ Na+共转运的第Ⅱ 类HKT转运蛋白的甘氨酸并非是决定其K+选择性吸收特性的唯一氨基酸残基, 因为一些Na+特异性转运的第Ⅰ 类HKT蛋白也具有K+转运特性, 且赖氨酸和精氨酸也参与调控其K+转运活性[55]。此外, 亚族Ⅰ 大部分成员在第2个P环区域的保守位置氨基酸是谷氨酰胺Asn(N), 当N突变为Asp(D)后, 成员的Na+转运特性转变为Na+∶ K+共转运特性, 这在拟南芥AtHKT1; 1、盐芥TsHKT1; 2及Eutrema parvula EpHKT1; 1中均得到验证[56, 57, 58]。
HKT家族成员定位在根表皮细胞膜及根、茎、叶鞘木质部薄壁细胞膜等处。研究表明, 亚族Ⅰ 成员野大麦HvHKT1; 1、玉米ZmHKT1、水稻OsHKT1; 4定位在木质部薄壁细胞膜上, 拟南芥AtHKT1; 1、番茄SlHKT1; 1和SlHKT1; 2、契斯曼尼番茄(Solanum cheesmaniae)ScHKT1; 1和ScHKT1; 2定位于木质部薄壁细胞的质膜和韧皮部上[59, 60, 61, 62]。Rosas-Santiago等[63]却发现, 水稻OsHKT1; 3定位在高尔基体上。亚族Ⅱ 成员冰叶日中花McHKT2、水稻OsHKT2; 1和OsHKT2; 2/1均定位在质膜上[64, 65]。
HKT家族成员在不同植物中的表达部位和调控效应不同(表3)。番茄和契斯曼尼番茄中的Sl(Sc)HKT1; 1和Sl(Sc)HKT1; 2在根、茎和叶中均有表达, 当遭受盐胁迫时, 根中Sl(Sc)HKT1; 1和Sl(Sc)HKT1; 2的表达显著上调, 而叶和茎中Sl(Sc)HKT1; 2的表达却下降[62]。小麦TaHKT2; 1和TaHKT2; 2在叶片、叶鞘和根中均有表达[66]。水稻、大麦、芦苇、小花碱茅(Puccinellia tenuiflora)等植物中, HKT2; 1在叶中有高表达, 根中表达量较低[51, 56, 57, 67, 68, 69]。水稻中的HKT家族成员最多, 目前在粳稻品种日本晴(Nipponbare)中鉴定到9个HKT成员, 其中5个为亚族Ⅰ 成员:OsHKT1; 1主要在叶片韧皮部表达, 受盐胁迫的显著诱导, 还受转录因子如MYBc等的调节[70]; OsHKT1; 3在叶、叶鞘及茎基部维管束中均有表达, 在韧皮部中的表达量很高; OsHKT1; 4主要在地上部维管束组织中表达[64]; OsHKT1; 5在根部和叶鞘木质部薄壁细胞膜上均有表达, 在成熟叶片韧皮部中也可观测到表达, 受盐胁迫的诱导[71]。亚族Ⅱ 成员OsHKT2; 1在水稻叶片的维管组织、近轴表皮和叶肉细胞中均可观测到表达[64, 72, 73]。水稻Nona Bokra品种No-OsHKT2; 2/1主要在根中表达, 受外界高Na+浓度的显著诱导[67]。OsHKT2; 3水稻在根、茎基部、叶和叶鞘中均有表达。OsHKT2; 4则在水稻根表皮细胞及茎、节间、叶鞘的维管束木质部和韧皮部中均有表达[74]。
| 表3 不同高等植物中的HKT家族成员 Table 3 Members of HKT family in different plants |
HKT家族亚族Ⅰ 成员多为Na+转运蛋白, 但一些成员也具有K+转运特性(表3)。拟南芥athkt1; 1突变体中的Na+内流不受盐胁迫影响, 但盐胁迫引起体内Na+的分配发生改变, Na+更多地积累在植株的地上部中, 且地上部中韧皮部Na+含量下降, 而木质部Na+含量增加[75]。Zhang等[76]发现, 枯草芽孢杆菌GB03通过抑制拟南芥根中AtHKT1; 1的表达降低整株的Na+积累。最近的研究表明, 盐胁迫下, AtHKT1; 1通过茎中木质部汁液Na+的回流, 减少Na+向花器官的运输, 从而提高植株的耐盐性[77]。Busoms等[78]进一步的研究表明, AtHKT1; 1的演化在拟南芥适应动态变化的盐浓度过程中起着至关重要的作用, 两种具有不同表达模式的AtHKT1; 1是使得各种拟南芥生态型在不同盐浓度条件下生存的最直接因素。可见, AtHKT1; 1参与拟南芥Na+的转运及稳态平衡过程。此外, 拟南芥sos3突变体中AtHKT1; 1的突变可显著缓解低K+胁迫下sos3根生长受抑的表型, 而AtHKT1; 1的超表达使其生长受抑更为显著, 表明, AtHKT1; 1也可能参与调节植物体内K+稳态平衡[79]。 盐芥TsHKT1; 2与AtHKT1; 1有较高的同源性, 但它仅具有K+转运特性, 赤桉EcHKT1; 2与TsHKT1; 2类似。玉米ZmHKT1功能缺失后会引起木质部Na+浓度的增加, 导致根向地上部Na+转运的增加, 表明ZmHKT1可促进叶中Na+的外排及木质部中Na+的卸载, 从而维持植株的耐盐性[61]。Han等[59]对西藏野生大麦的研究表明, HvHKT1; 1基因的敲除会引起根系和叶片中的Na+积累增加, 而将HvHKT1; 1过表达在盐敏感拟南芥hkt1; 4和sos1-12突变株中, 可显著降低根和地上部中的Na+含量, 表明, HvHKT1; 1在根中Na+的转运过程中发挥重要作用。HKT家族亚族Ⅱ 成员不仅具有K+∶ Na+共转运特性, 在外界环境的影响下, 还可作为K+、Na+单向转运体(表3)。小麦TaHKT2; 1属于K+∶ Na+共转运蛋白, 当外界Na+浓度较低时, 同时介导K+、Na+吸收, 是一种Na+藕联的K+转运蛋白, 但当外界低K+高Na+时, 仅介导低亲和性Na+吸收[80]。Laurie等[81]将TaHKT2; 1以反义方式转进小麦, 在低K+和高Na+下检测膜的去极化, 发现低K+下转基因植株和对照株没有差异, 而高Na+转基因植株比对照去极化程度快, 且高Na+(200 mmol· L-1)处理下, 转基因植株体内积累的Na+含量降低, 耐盐性远高于对照, 进一步表明小麦根中TaHKT2; 1主要介导Na+内流。相似地, 在爪蟾卵母细胞中, 水稻OsHKT2; 1仅在外界毫摩尔Na+(mmol· L-1)浓度范围内发挥Na+∶ K+共转运活性, 当外界Na+浓度较高时, 只转运N
Shaker家族是第一个在分子水平上被确定的植物K+通道, 它是通过酵母吸钾双突变体互补法从拟南芥cDNA文库中分离出来的[91, 92]。Sentenac等[91]和Anderson等[92]从拟南芥中首次克隆到K+通道基因KAT1和AKT1。Ché rel等[93]进一步分析发现, KAT1和AKT1与动物K+通道的功能和结构相似。Shaker家族可划分为5个亚族[94]。亚族Ⅰ 和Ⅱ 由5个内整流K+通道组成(AKT1, SPIK, AKT6, KAT1和KAT2), 亚族Ⅰ 和Ⅱ 的成员可通过结构上是否含有锚蛋白区和C末端得以区分。亚族Ⅲ 由单一的成员AKT2组成, 含有锚蛋白区。亚族Ⅳ 由单一成员AtKC1组成, 不含锚蛋白区域。亚族Ⅴ 由两个外整流K+通道GORK和SKOR组成, 受激活的电压范围和整流特性与内整流通道相反, 含有锚蛋白区域。
Shaker通道是多亚基蛋白, 由4个ɑ -亚基组成。在四聚物结构中, 这4个亚基可以是一个Shaker基因的产物, 也可以是不同Shaker基因的产物。这些亚基拥有确定的位置, 每一个亚基由6个跨膜结构(TM1-TM6)组成疏水区域, 含有N-和C-末端区域[91]。TM5和TM6之间含有一个高度保守呈β 发夹状的P环结构。P环结构作为离子介导中心选择性过滤区域, 其所特有的TxxTxGYGD/E正是K+通道标志性序列, 该结构对于K+具有高度选择性(表1)[93]。TM4由带正电荷的氨基酸残基组成, 能感应跨膜电压的变化, 进而控制内整流K+通道的开闭[95]。C-末端在胞质内, 主要由环核苷酸结合区域(cNMP)、锚蛋白(ankyrin)重复域和富含疏水酸性残基区(KHA domain)组成[96]。
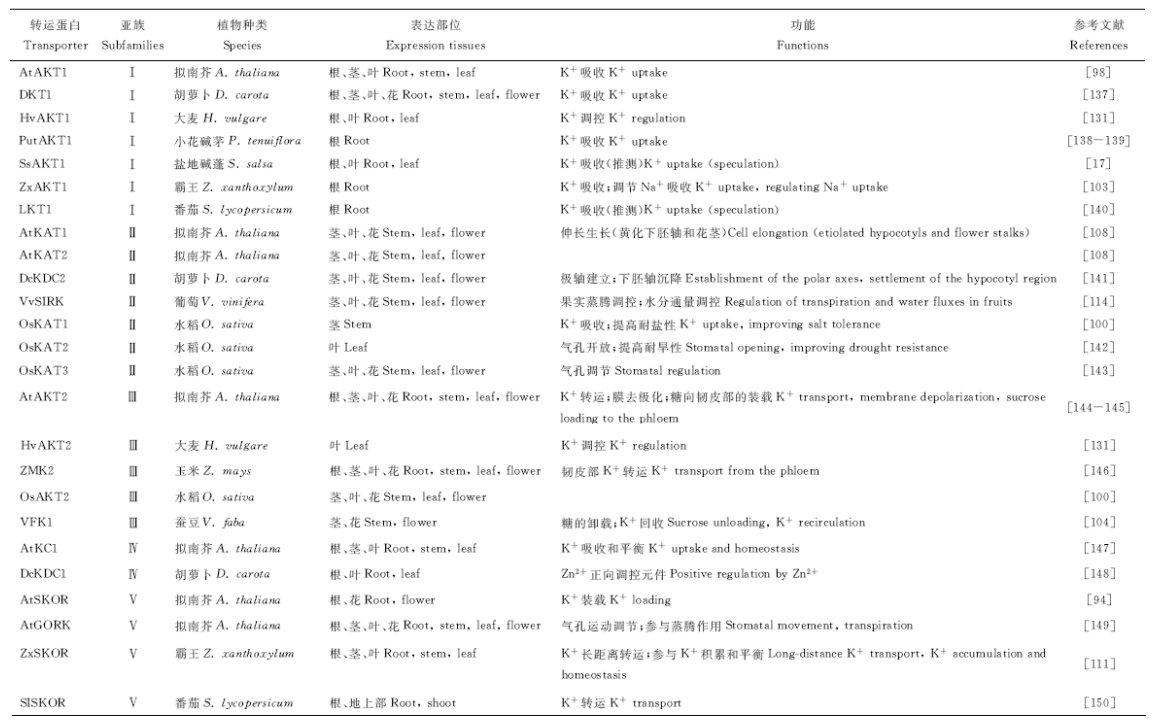
Shaker家族大部分成员均定位在质膜上。亚族Ⅰ 的成员如拟南芥AtAKT1、霸王(Zygophyllum xanthoxylum)ZxAKT1、葡萄VvK1.1、番茄LKT1、胡萝卜(Daucus carota)DKT1、盐地碱蓬(Suaeda salsa)SsAKT1等均定位在根的质膜上, 并主要在根表皮细胞中表达[17, 97](表4)。在其他部位也可发现该亚族成员Ⅰ 的表达, 如在下胚轴、叶原基细胞、水孔和保卫细胞中可观测到AtAKT1的表达[98], 在葡萄的根皮层、花、果实和种子中都有VvK1.2表达[99], 从玉米胚芽鞘中分离出的ZMK1, 在根皮层、花粉中也均有表达[100]。然而, AtSPIK和AtAKT6主要在拟南芥花中表达, 马铃薯(Solanum tuberosum)SKT1却仅在保卫细胞中表达[101, 102]。霸王ZxAKT1优先在根中表达, 受介质中高浓度KCl或NaCl的诱导[103]。亚族Ⅱ 的成员拟南芥AtKAT1定位在子叶和下胚轴的保卫细胞中, 在黄化下胚轴皮层、表皮和花茎中也有分布, 而AtKAT2特异地分布在子叶和黄化幼苗顶端的韧皮部中, AtKAT1和AtKAT2的表达均受植物激素的显著诱导。类似地, 甜瓜(Cucumis melo)MIRK、玉米ZmK2.1和KZM2等[4]亚族Ⅱ 的成员特异地表达在韧皮部中, 在植物的地上部组织中(如茎、叶和花)表达。亚族Ⅲ 的成员几乎都在韧皮部表达, 如拟南芥AtAKT2、蚕豆(Vicia faba)VFKT1和杨树PTK2等[101, 104]。单子叶植物中, 关于亚族Ⅲ 基因成员表达模式分析的研究报道很少, 其中玉米ZMK2被报道在胚芽鞘、中胚轴和叶中表达, 也表现出韧皮部的表达特性[105]。除了拟南芥AtKC1, 在胡萝卜中也已经鉴定到KDC1, 这两个基因都属于亚族Ⅳ 成员, 在根皮层尤其根毛中有高表达[106, 107]。亚族Ⅴ 的成员具有不同的表达模式, 如拟南芥AtSKOR在根中柱组织和花粉中表达[102]; 拟南芥AtGORK则在保卫细胞、根毛和下胚轴中表达[108]; 玉米ZORK在保卫细胞中表达[109]; 杨树PTORK在保卫细胞、叶片木质部中都观察到了表达[110]; ZxSKOR在霸王的根、茎和叶中都有表达, 其中在根中的表达量最高, 受Na+的显著诱导[111]。
| 表4 不同高等植物中的Shaker家族成员 Table 4 Members of Shaker family in different plants |
Shaker选择性K+通道受电压调控, 根据受激活的电压范围及整流特性, 可分为3类:内向整流通道(inward rectifier, IR)(包括Ⅰ 、Ⅱ 亚族)、弱内向整流通道(weak inward rectifier, WIR)(包括第Ⅲ 亚族)和外向整流通道(outward rectifier, OR)(包括亚族Ⅴ )[112]。亚族Ⅰ 和Ⅱ 成员受反向电压激活, 具有低K+转运亲和性, 且大部分成员的K+转运活性受pH值调节, 外部介质的酸化会增加电流水平和电导率[93]。亚族Ⅰ 的一些成员允许N
很多研究表明, 大量调控因子参与质膜中植物Shaker通道的调控或活性激活过程, 包括β -亚基、14-3-3蛋白、不同种类的激酶、磷酸酶和SNAREs等[1]。拟南芥中, 应对盐胁迫和K+缺失的信号网络中, 有26个丝氨酸/苏氨酸CIPKs(protein kinases)和10个CBLs(calcineurin B-like)参与植物对环境信号的响应[10]。Ca2+与CBLs的结合需要CIPK-CBL的相互作用才能完成。上游的CBL1和CBL9可与CIPK23相互作用, 将CIPK23锚定于细胞质膜上, 随后CIPK23对Shaker K+通道AKT1进行磷酸化, 激活AKT1的K+转运活性, 促进植物在低K+条件下吸收
相同亚族成员在不同的植物种类中具有不同的生理功能。Ahmad等[132]对比了水稻OsAKT1缺失突变体和超表达突变体后发现, OsAKT1的过表达可以显著增加水稻组织中尤其是根中的K+含量, 从而提高水稻的渗透调节能力, 以抵御干旱胁迫。经异源表达系统鉴定, 玉米ZmK2.1受外界K+浓度的强烈调节, 与膜的超级化无关, 在外界Km值15 mmol· L-1左右时转运活性被激活, 无论膜电位如何, 均不参与亚微摩尔级的K+吸收[133]。研究表明, 玉米保卫细胞中还存在如ZMK2类不受外界K+浓度影响的内整流K+通道, 这样玉米保卫细胞可在较大的K+浓度范围内对K+产生响应[134]。然而, 拟南芥的保卫细胞中仅有KAT1内整流K+通道基因表达。可见, 不同植物保卫细胞中内整流K+通道的多样性会引起它们功能方面产生很大差异。耐盐甜瓜品种中, 内整流K+通道MIRK在保卫细胞中表达, 其介导的K+电流受外界Na+的抑制, 从而影响盐胁迫下的K+转运[135]。此外, MIRK可能通过Na+引起的气孔开闭控制Na+向地上部组织中的运输, 表明内整流K+通道可能参与保卫细胞中除调控气孔开闭以外的其他重要生理过程[136]。
Nieves-Cordones等[9]对比分析了拟南芥和水稻体内的K+吸收及转运过程:拟南芥体内参与K+吸收的主要成员是AtHAK5和AtAKT1, AtKUP7也可介导K+吸收, K+向木质部的释放主要由AtSKOR介导, AtKUP7在此过程中也可发挥一定的作用; 水稻体内OsAKT1、OsHAK1和OsHAK5共同参与K+的吸收, 此外, 这3类转运系统可能直接或间接发挥作用, 促进K+向木质部的释放。ZxAKT1参与霸王体内主要的K+吸收过程, ZxSKOR参与霸王体内K+的长距离运输, 在盐胁迫下, 当霸王体内ZxAKT1被干扰掉之后, ZxSKOR的表达显著下降, 且ZxSOS1、ZxHKT1; 1、ZxNHX在根和地上部中的表达均显著下降, 表明ZxAKT1不仅在霸王的K+吸收过程中发挥重要作用, 还参与调控Na+的吸收及转运[103, 111]。可见, 不同植物体内存在特有的K+吸收及转运系统, 且系统内各成员之间可产生相互影响。
KT/HAK/KUP家族转运蛋白、HKT家族转运蛋白和Shaker通道是参与植物体内K+吸收和转运的主要蛋白和通道, 目前, 已从多种植物中展开了相关蛋白的作用机理和调控机制研究, 但仍有许多问题尚未解决。如:其一, 虽然研究表明HKT家族在植物K+、Na+转运平衡的过程中具有重要的生理功能, 但对其作用机制尚无定论, 且不同物种间存在显著差异。对于这一问题, 今后在深入挖掘基因功能的同时, 应着重加强不同植物之间的共性和特异性分析。其二, KT/HAK/KUP家族成员的结构目前还不清楚, 成员是否参与植物细胞中K+信号传递和区域化也属未知, 且多基因家族中, 想要全面认识其功能需要进一步研究, 成员之间功能上的协作关系是否存在, 如果确实存在, 那协同如何发生及协同作用对植物生理功能的影响都是非常值得探讨的问题。其三, Shaker通道的基因受上下游响应基因和外界各种因素的调节, 充分了解成员的表达模式与生理功能为上游调节基因的信号转导途径研究可提供很好的途径。因此, 建议通过基因组学、转录组学、蛋白组学、代谢组学、全基因组甲基化学等方法系统深入的研究植物体内K+吸收及转运的分子机制, 加大对相关基因生理功能的探究; 从具有代表性的不同类型植物中展开相关基因的研究, 通过基因沉默、过表达等方法进行功能的深入验证, 为作物遗传改良提供理论依据; 建议推动建立相关的专用数据库, 将学者们对K+吸收及转运相关基因的序列特征、表达模式、功能和调控等的研究成果进行综合整理, 以便得到更好地理解和应用。
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|