

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2023, Vol. 32 ›› Issue (8): 202-213.DOI: 10.11686/cyxb2022417
• 综合评述 • 上一篇
孙守江( ), 毛培胜(
), 毛培胜( ), 豆丽茹, 贾志程, 孙铭, 马馼, 欧成明, 王娟
), 豆丽茹, 贾志程, 孙铭, 马馼, 欧成明, 王娟
收稿日期:2022-10-18
修回日期:2023-01-10
出版日期:2023-08-20
发布日期:2023-06-16
通讯作者:
毛培胜
作者简介:E-mail: maops@cau.edu.cn基金资助:
Shou-jiang SUN( ), Pei-sheng MAO(
), Pei-sheng MAO( ), Li-ru DOU, Zhi-cheng JIA, Ming SUN, Wen MA, Cheng-ming OU, Juan WANG
), Li-ru DOU, Zhi-cheng JIA, Ming SUN, Wen MA, Cheng-ming OU, Juan WANG
Received:2022-10-18
Revised:2023-01-10
Online:2023-08-20
Published:2023-06-16
Contact:
Pei-sheng MAO
摘要:
种子使植物能够在恶劣的环境条件下生存,将遗传信息从亲代传递给下一代。种子活力是农业中的一个重要性状,直接影响田间出苗率和作物产量。然而,由于种子老化,种子活力在贮藏过程中下降。为了有效地保护基因资源,减少由于种子老化给农业生产带来的巨大经济损失,有必要探究种子老化的机制,以便了解种子老化的起因以及老化过程中发生的一系列重要事件。在种子贮藏过程中,高温高湿是加速种子老化的两个主要因素。活性氧(ROS)引起的氧化损伤是主要原因。ROS可导致蛋白质损伤、脂质过氧化、染色体端粒结构异常和DNA损伤引起的各种细胞成分损伤。此外,ROS还诱导细胞程序性死亡,导致种子老化。种子在吸胀初期会对一些损伤进行修复,但是,如果对关键结构造成巨大损伤,就会导致修复失败,种子永久失去活力,无法在相对较短的时间内正常发芽。种子老化的确切机制尚未得到全面研究。基于此,本研究主要综述了种子老化过程中ROS的产生以及清除途径、ROS对生物大分子的影响、染色体端粒系统对种子老化的响应以及种子耐老化相关基因的研究进展,并提出了展望。这对了解种子老化的原因以及种子老化机制的解析具有重要意义。
孙守江, 毛培胜, 豆丽茹, 贾志程, 孙铭, 马馼, 欧成明, 王娟. 活性氧及染色体端粒调控种子老化研究[J]. 草业学报, 2023, 32(8): 202-213.
Shou-jiang SUN, Pei-sheng MAO, Li-ru DOU, Zhi-cheng JIA, Ming SUN, Wen MA, Cheng-ming OU, Juan WANG. Studies on the regulation of seed aging by reactive oxygen species and telomeres[J]. Acta Prataculturae Sinica, 2023, 32(8): 202-213.
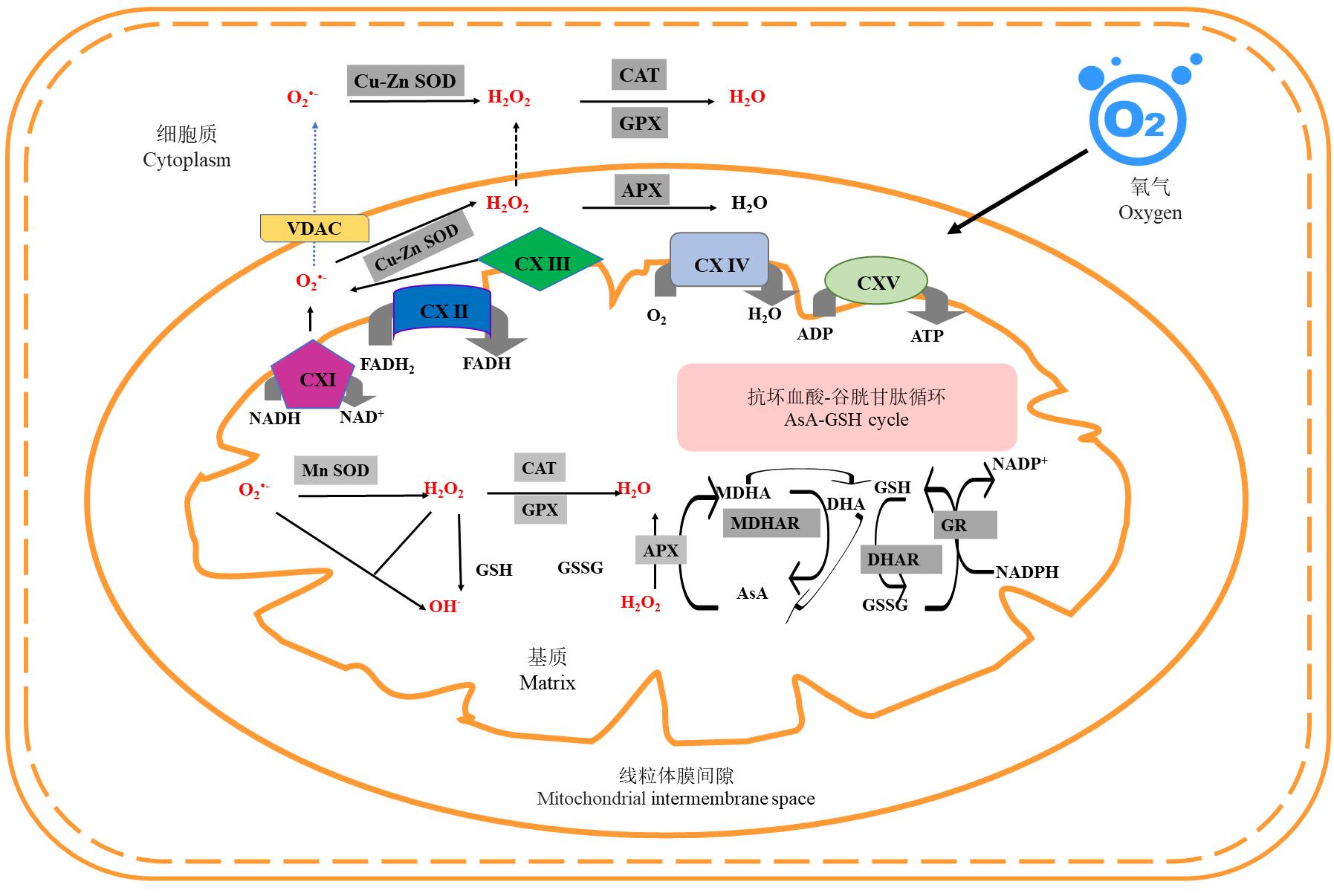
图1 种子老化过程中活性氧的产生和清除CXI、CXII、CXIII、CXIV、 CXV、VDAC、CAT、GPX、APX、NADH、NAD+、FADH2、FADH、ADP、ATP、GSH、GSSG、MDHAR、DHAR、AsA、DHA、NADP+和NADPH分别表示NADH-Q还原酶、琥珀酸-Q还原酶、细胞色素C还原酶、细胞色素C氧化酶、ATP合成酶、电压依赖性阴离子通道、过氧化氢酶、谷胱甘肽过氧化物酶、抗坏血酸过氧化物酶、还原型烟酰胺腺嘌呤二核苷酸、氧化型烟酰胺腺嘌呤二核苷酸、还原型黄素二核苷酸、氧化型黄素二核苷酸、腺嘌呤核苷二磷酸、腺嘌呤核苷三磷酸、还原型谷胱甘肽、氧化型谷胱甘肽、单脱氢抗坏血酸还原酶、脱氢抗坏血酸还原酶、抗坏血酸、氧化型抗坏血酸、烟酰胺腺嘌呤二核苷酸磷酸和还原型烟酰胺腺嘌呤二核苷酸磷酸。下同。CXI, CXII, CXIII, CXIV, CXV, VDAC, CAT, GPX, APX, NADH, NAD+, FADH2, FADH, ADP, ATP, GSH, GSSG, MDHAR, DHAR, AsA, DHA, NADP+ and NADPH represent NADH-Q reductase, succinic acid-Q reductase, cytochrome C reductase, cytochrome C oxidase, ATP synthetase, voltage-dependent anion channel, catalase, glutathione peroxidase, ascorbate peroxidase, reduced nicotinamide adenine dinucleotide, oxidized nicotinamide adenine dinucleotide, reduced flavin dinucleotide, oxidized flavin dinucleotide, adenosine diphosphate, adenosine triphosphate, reduced glutathione, oxidized glutathione, monodehydroascorbic reductase, dehydroascorbic reductase, ascorbic acid, oxidized ascorbic acid, niacinamide adenine dinucleotide phosphate and reduced nicotinamide adenine dinucleotide phosphate. The same below.
Fig. 1 Production and elimination of reactive oxygen species during seed aging

图3 细胞程序性死亡中ROS依赖的信号传导ROS、MCU、ANT和φm分别表示活性氧、线粒体钙单向转运体、腺嘌呤核苷酸转运体和线粒体膜电势。ROS, MCU, ANT and φm represent reactive oxygen species, calcium uniporter, adenine nucleotide translocator and mitochondrial membrane potential, respectively.
Fig.3 ROS-dependent signaling involved in programmed cell death
| 1 | Baskin C C, Baskin J M. Breaking seed dormancy during dry storage: A useful tool or major problem for successful restoration via direct seeding? Plants, 2020, 9(5): 636-649. |
| 2 | Ebone L A, Caverzan A, Chavarria G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiology and Biochemistry, 2019,145: 34-42. |
| 3 | Harman D. Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology, 1956, 11(3): 298-300. |
| 4 | Xue X, Zhang Q, Wu J X. Research of reactive oxygen species in plants and its application on stress tolerance. Biotechnology Bulletin, 2013(10): 6-11. |
| 薛鑫, 张芊, 吴金霞. 植物体内活性氧的研究及其在植物抗逆方面的应用. 生物技术通报, 2013(10): 6-11. | |
| 5 | Ratajczak E, Małecka A, Ciereszko I, et al. Mitochondria are important determinants of the aging of seeds. International Journal of Molecular Sciences, 2019, 20(7): 1568-1572. |
| 6 | Domergue J B, Abadie C, Limami A, et al. Seed quality and carbon primary metabolism. Plant, Cell and Environment, 2019, 42(10): 2776-2788. |
| 7 | Wang Y, Li Y, Xue H, et al. Reactive oxygen species-provoked mitochondria-dependent cell death during ageing of elm (Ulmus pumila L.) seeds. The Plant Journal, 2015, 81(3): 438-452. |
| 8 | Oenel A, Fekete A, Krischke M, et al. Enzymatic and non-enzymatic mechanisms contribute to lipid oxidation during seed aging. Plant Cell Physiology, 2017, 58: 925-933. |
| 9 | Rajjou L, Debeaujon I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biologies, 2008, 331(10): 796-805. |
| 10 | Murthy U, Kumar P P, Sun W Q. Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: Lipid peroxidation, sugar hydrolysis, maillard reactions and their relationship to glass state transition. Journal of Experimental Botany, 2003, 54: 1057-1067. |
| 11 | Hu D, Ma G, Wang Q, et al. Spatial and temporal nature of reactive oxygen species production and programmed cell death in elm (Ulmus pumila L.) seeds during controlled deterioration. Plant, Cell & Environment, 2012, 35(11): 2045-2059. |
| 12 | Wang W Q, Xu D Y, Sui Y P, et al. A multiomic study uncovers a bZIP23-PER1A-mediated detoxification pathway to enhance seed vigor in rice. Proceedings of the National Academy of Sciences of the United States of America, 2022(9): 119-132. |
| 13 | Bailly C. Active oxygen species and antioxidants in seed biology. Seed Science Research, 2004, 14(2): 93-107. |
| 14 | Waszczak C, Carmody M, Kangasjarvi J. Reactive oxygen species in plant signaling. Annual Review of Plant Biology, 2018, 69: 209-236. |
| 15 | Xu F, Yuan S, Liang H G, et al. The roles of alternating oxidase and uncoupling protein in plant mitochondria and their interrelationships. Plant Physiology Journal, 2009, 45(2): 105-110. |
| 徐飞, 袁澍, 梁厚果, 等. 交替氧化酶和解偶联蛋白在植物线粒体中的作用及其相互关系. 植物生理学报, 2009, 45(2): 105-110. | |
| 16 | McDonald A E. Alternative oxidase: An inter-kingdom perspective on the function and regulation of this broadly distributed ‘cyanide-resistant’ terminal oxidase. Functional Plant Biology, 2008, 35(7): 535-552. |
| 17 | Cvetkovska M, Vanlerberghe G C. Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytologist, 2012, 195(1): 32-39. |
| 18 | Clifton R, Millar A H, Whelan J. Alternative oxidases in Arabidopsis: A comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochimica et Biophysica Acta(BBA)-Bioenergetics, 2006, 1757(7): 730-741. |
| 19 | Bewley J D, Bradford K, Hilhorst H. Seeds: Physiology of development, germination and dormancy. Springer Science & Business Media, 2012. |
| 20 | Vanlerberghe G. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. International Journal of Molecular Sciences, 2013, 14(4): 6805-6847. |
| 21 | Wang H, Ma Y W, Qiao Z H. Structural and functional characterization of AOX gene family. Biotechnology Bulletin, 2022, 38(7): 160-170. |
| 王慧, 马艺文, 乔正浩. AOX基因家族的结构和功能特征分析. 生物技术通报, 2022, 38(7): 160-170. | |
| 22 | Hu Y S, Gao W R, Wang R F, et al. Analysis of AOX and UCP families’ expression level in Prunus auriculata under stress. Forestry Science & Technology, 2015, 40(1): 6-10. |
| 胡银松, 高文蕊, 王瑞芳, 等. 胁迫下欧李AOX及UCP基因家族表达分析. 林业科技, 2015, 40(1): 6-10. | |
| 23 | Wei Y, Wang X, Shao X, et al. Sucrose treatment of mung bean seeds results in increased vitamin C, total phenolics, and antioxidant activity in mung bean sprouts. Food Science and Nutrition, 2019, 7(12): 4037-4044. |
| 24 | Camacho-Pereira J, Meyer L E, Machado L B, et al. Reactive oxygen species production by potato tuber mitochondria is modulated by mitochondrially bound hexokinase activity. Plant Physiology, 2009, 149(2): 1099-1110. |
| 25 | Wang J H, Liu H X, Xu T. The role of superoxide dismutase (SOD) in stress physiology and senescence physiology of plant. Plant Physiology Journal, 1989, 1(1): 1-7. |
| 王建华, 刘鸿先, 徐同. 超氧物歧化酶(SOD)在植物逆境和衰老生理中的作用. 植物生理学报, 1989, 1(1): 1-7. | |
| 26 | Zhang W W, Zheng F X, Wang X K, et al. Effects of ozone on root activity, soluble protein content and antioxidant system in Oryza sativa roots. Chinese Journal of Plant Ecology, 2009, 33(3): 425-432. |
| 张巍巍, 郑飞翔, 王效科, 等. 臭氧对水稻根系活力、可溶性蛋白含量与抗氧化系统的影响. 植物生态学报, 2009, 33(3): 425-432. | |
| 27 | Jimenez A, Hernandez J A, Del Rio L A, et al. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiology, 1997, 114(1): 275-284. |
| 28 | Linster C L, Adler L N, Webb K, et al. A second GDP-L-galactose phosphorylase in Arabidopsis en route to vitamin C. Journal of Biological Chemistry, 2008, 283(27): 18483-18492. |
| 29 | Farmer E E, Mueller M J. ROS-mediated lipid peroxidation and RES-activated signaling. Annual Review of Plant Biology, 2013, 64: 429-450. |
| 30 | Min C W, Lee S H, Cheon Y E, et al. In depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. Journal of Proteomics, 2017, 169: 125-135. |
| 31 | Lee H C, Wei Y H. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Experimental Biology and Medicine, 2007, 232(5): 592-606. |
| 32 | Satour P, Youssef C, Chatelain E, et al. Patterns of protein carbonylation during Medicago truncatula seed maturation. Plant Cell Environment, 2018, 41(9): 2183-2194. |
| 33 | Boucelha L, Abrous B O, Djebbar R. Is protein carbonylation a biomarker of seed priming and ageing? Functional Plant Biology, 2021, 48(6): 611-623. |
| 34 | Sano N, Rajjou L, North H M, et al. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiology, 2016, 57(4): 660-674. |
| 35 | Kranner I, Birti S, Anderson K M, et al. Glutathione half-cell reduction potential: A universal stress marker and modulator of programmed cell death? Free Radical Biology and Medicine, 2006, 40(12): 2155-2165. |
| 36 | Kranner I, Chen H, Pritchard H W, et al. Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed aging. Plant Growth Regulation, 2011, 63: 63-72. |
| 37 | Mao C, Zhu Y, Cheng H, et al. Nitric oxide regulates seedling growth and mitochondrial responses in aged oat seeds. International Journal Molecular Science, 2018, 19(4): 1052-1065. |
| 38 | Roberts J, Florentine S, Etten E V, et al. Seed longevity and germination in response to changing drought and heat conditions on four populations of the invasive weed African lovegrass (Eragrostis curvula). Weed Science, 2021, 69(4): 1-25. |
| 39 | Waterworth W M, Footitt S, Bray C M, et al. DNA damage checkpoint kinase ATM regulates germination and maintains genome stability in seeds. Proceedings of the National Academy of Sciences, 2016, 113(34): 9647-9652. |
| 40 | Waterworth W M, Masnavi G, Bhardwaj R M, et al. A plant DNA ligase is an important determinant of seed longevity. Plant Journal, 2010, 63(5): 848-860. |
| 41 | Chen H H, Chu P, Zhou Y L, et al. Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. Journal of Experimental Botany, 2012, 63(11): 4107-4121. |
| 42 | Costa-Nunes J, Bhatt A M, O'Shea S, et al. Characterization of the three Arabidopsis thaliana RAD21 cohesins reveals differential responses to ionizing radiation. Journal of Experimental Botany, 2006, 57(4): 971-983. |
| 43 | Osborne D J. Hazards of a germinating seed: Available water and the maintenance of genomic integrity. Israel Journal of Plant Sciences, 2000, 48(3): 173-179. |
| 44 | Balestrazzi A, Confalonieri M, Macovei A, et al. Seed imbibition in Medicago truncatula Gaertn.: Expression profiles of DNA repair genes in relation to PEG-mediated stress. Journal of Plant Physiology, 2011, 168(7): 706-713. |
| 45 | Hunt L, Holdsworth M J, Gray J E. Nicotinamidase activity is important for germination. The Plant Journal, 2007, 51(3): 341-351. |
| 46 | Miquel J, Economos A C, Fleming J, et al. Mitochondrial role in cell aging. Experimental Gerontology, 1980, 15(6): 575-591. |
| 47 | Greenberg J. A taxonomy of organizational justice theories. Academy of Management Review, 1987, 12(1): 9-22. |
| 48 | Bucholc M, Buchowicz J. Synthesis of extrachromosomal DNA and telomere-related sequences in germinating wheat embryos. Seed Science Research, 1992, 2(3): 141-146. |
| 49 | Donà M, Balestrazzi A, Mondoni A, et al. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Annals of Botany, 2013, 111(5): 987-998. |
| 50 | Zhang K L, Zhang Y, Sun J, et al. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiology and Biochemistry, 2021, 158: 475-485. |
| 51 | Chen B, Yin G, Whelan J, et al. Composition of mitochondrial complex I during the critical node of seed aging in Oryza sativa. Journal of Plant Physiology, 2019, 236: 7-14. |
| 52 | Chen H H, Chu P, Zhou Y L, et al. Ectopic expression of NnPER1, a Nelumbo nucifera 1‐cysteine peroxiredoxin antioxidant, enhances seed longevity and stress tolerance in Arabidopsis. The Plant Journal, 2016, 88(4): 608-619. |
| 53 | Jiang X C, Zhou S Q. Research progress on molecular mechanisms of seed vigor and anti-aging ability. Life Science Research, 2021, 25(5): 406-416. |
| 姜孝成, 周诗琪. 种子活力或抗老化能力的分子机制研究进展. 生命科学研究, 2021, 25(5): 406-416. | |
| 54 | Nagel M, Kodde J, Pistrick S, et al. Barley seed aging: Genetics behind the dry elevated pressure of oxygen aging and moist controlled deterioration. Frontiers in Plant Science, 2016, 7: 388-395. |
| 55 | Gayen D, Ali N, Ganguly M, et al. RNAi mediated silencing of lipoxygenase gene to maintain rice grain quality and viability during storage. Plant Cell, Tissue and Organ Culture, 2014, 118(2): 229-243. |
| 56 | Xu H, Wei Y, Zhu Y, et al. Antisense suppression of LOX3 gene expression in rice endosperm enhances seed longevity. Plant Biotechnology Journal, 2015, 13(4): 526-539. |
| 57 | Li B B. Analysis of seed storage tolerance of wheat (Triticum aestivum) lipoxygenase RNAi transgenic lines. Xianyang: Northwest A & F University, 2016. |
| 李冰冰. 小麦转LOXi基因后代外源基因稳定性检测及种子耐储藏性分析. 咸阳: 西北农林科技大学, 2016. | |
| 58 | Huang J, Cai M, Long Q, et al. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Research, 2014, 23(4): 643-655. |
| 59 | Qian W, Kumar N, Roginskaya V, et al. Chemoptogenetic damage to mitochondria causes rapid telomere dysfunction. Proceedings of the National Academy of Sciences, 2019, 116(37): 18435-18444. |
| [1] | 柳文蔚, 刘鑫, 雷映霞, 周青平, 刘志峰, 王沛. 老芒麦种质资源抗寒性综合评价及冷胁迫下的生理反应[J]. 草业学报, 2023, 32(8): 152-163. |
| [2] | 高子奇, 王佳, 汤宇晨, 王迎春. 唐古特白刺类黄酮-3-O-葡萄糖基转移酶基因(NtUFGT)的克隆与功能分析[J]. 草业学报, 2020, 29(5): 159-170. |
| [3] | 贾茵, 向元芬, 王琳璐, 赵健, 刘才磊, 潘远智. 盐胁迫对小报春生长及生理特性的影响[J]. 草业学报, 2020, 29(10): 119-128. |
| [4] | 刘建新, 欧晓彬, 王金成, 刘瑞瑞, 贾海燕. 镉胁迫下裸燕麦幼苗对外源H2O2的生理响应[J]. 草业学报, 2020, 29(1): 125-134. |
| [5] | 孙铭, 王思琪, 艾尔肯·达吾提, 毛培胜. 抗氧化剂引发对无芒雀麦老化种子发芽及幼苗生长的影响[J]. 草业学报, 2019, 28(11): 105-113. |
| [6] | 夏方山, 董秋丽, 毛培胜, 王明亚, 陈玲玲, 程航. PEG引发对老化燕麦种胚细胞与线粒体结构及抗氧化性能的影响[J]. 草业学报, 2018, 27(5): 170-177. |
| [7] | 刘建新, 欧晓彬, 王金成, 刘秀丽. 过氧化氢提高燕麦幼苗耐碱性的活性氧代谢和渗透调节[J]. 草业学报, 2018, 27(2): 97-104. |
| [8] | 刘建新, 王金成, 刘秀丽. 外源NO对镧胁迫下燕麦幼苗活性氧代谢和矿质元素含量的影响[J]. 草业学报, 2017, 26(5): 135-143. |
| [9] | 田沛, 南志标. 内生真菌与寄主互惠共生的分子机制[J]. 草业学报, 2017, 26(4): 196-210. |
| [10] | 史毅, 牛奎举, 余倩倩, 刘文辉, 马晖玲. 低温对青海扁茎早熟禾活性氧代谢的影响和相关基因表达分析[J]. 草业学报, 2017, 26(12): 98-107. |
| [11] | 马乐元, 陈年来, 韩国君, 李良, 孙小妹. 外源水杨酸对干旱胁迫下小冠花叶片活性氧水平及抗氧化系统的影响[J]. 草业学报, 2017, 26(10): 129-139. |
| [12] | 刘建新, 王金成, 王瑞娟, 贾海燕. 外源过氧化氢提高燕麦耐盐性的生理机制[J]. 草业学报, 2016, 25(2): 216-222. |
| [13] | 吴旭红, 吕成敏, 冯晶旻. 外源一氧化氮(NO)对低温胁迫下南瓜幼苗氧化损伤的保护效应[J]. 草业学报, 2016, 25(12): 161-169. |
| [14] | 张兰, 滕珂, 肖国增, 梁小红, 许立新, 尹淑霞, 晁跃辉. 日本结缕草ZjADH基因对拟南芥的转化及其耐寒性分析[J]. 草业学报, 2016, 25(11): 43-49. |
| [15] | 叶德友, 漆永红, 李敏权. 植物与线虫互作的信号传导及调控机制研究进展[J]. 草业学报, 2016, 25(10): 191-201. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||