

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2024, Vol. 33 ›› Issue (1): 198-206.DOI: 10.11686/cyxb2023089
• 综合评述 • 上一篇
孙守江( ), 马馼, 毛培胜(
), 马馼, 毛培胜( ), 豆丽茹, 贾志程, 孙铭, 王娟
), 豆丽茹, 贾志程, 孙铭, 王娟
收稿日期:2023-03-22
修回日期:2023-05-29
出版日期:2024-01-20
发布日期:2023-11-23
通讯作者:
毛培胜
作者简介:E-mail: maops@cau.edu.cn基金资助:
Shou-jiang SUN( ), Wen MA, Pei-sheng MAO(
), Wen MA, Pei-sheng MAO( ), Li-ru DOU, Zhi-cheng JIA, Ming SUN, Juan WANG
), Li-ru DOU, Zhi-cheng JIA, Ming SUN, Juan WANG
Received:2023-03-22
Revised:2023-05-29
Online:2024-01-20
Published:2023-11-23
Contact:
Pei-sheng MAO
摘要:
植物衰老和种子劣变机理的研究一直是农业科学领域关注的热点。植物衰老会对农业产生巨大的负面影响,牧草提前衰老也会导致草地生产力下降,限制草产业的发展。由于种子劣变,全球每年约有25%的种子失去活力,导致巨额的经济损失,严重影响农业的健康发展。深入揭示植物衰老特性和调控机制,不仅对于阐明植物生态适应性及种群稳定性具有重要价值,而且对于延缓衰老技术和调控措施的选择具有重要实践意义。在模式植物拟南芥研究中发现,染色体端粒与植物衰老以及种子活力密切相关。端粒是染色体末端的重复DNA序列,由端粒DNA和结合蛋白组成。端粒结合蛋白是一组与端粒DNA结合的蛋白质,主要是帮助稳定端粒结构并保护端粒免受DNA修复系统的干扰,其次还参与了基因表达、DNA复制和染色体结构调节等许多生物学过程。端粒酶由端粒酶逆转录酶(TERT)和端粒酶RNA(TER)两个亚单位组成,端粒酶逆转录酶亚基参与线粒体功能以及相关基因表达调控,通过对端粒酶新功能的探索,有助于提高植物的抗逆性,从而延缓植物的衰老进程,为提高作物产量提供一条新的途径。近年来在植物中研究发现,端粒的动态变化与植物衰老存在相关性,植物端粒内稳态的维持机制仍存在许多问题,端粒长度以及端粒酶活性的动态变化与植物衰老以及种子老化的关系尚不清楚。基于此,本研究综述了端粒和端粒结合蛋白在植物生物学中的作用,重点论述了端粒和端粒结合蛋白调控端粒长度以及端粒酶活性的模式,为后续解析植物衰老以及种子劣变机理提供理论参考依据。
孙守江, 马馼, 毛培胜, 豆丽茹, 贾志程, 孙铭, 王娟. 植物端粒DNA结合蛋白及端粒酶活性调控研究[J]. 草业学报, 2024, 33(1): 198-206.
Shou-jiang SUN, Wen MA, Pei-sheng MAO, Li-ru DOU, Zhi-cheng JIA, Ming SUN, Juan WANG. Regulation of telomere DNA binding protein and telomerase activity in plants[J]. Acta Prataculturae Sinica, 2024, 33(1): 198-206.
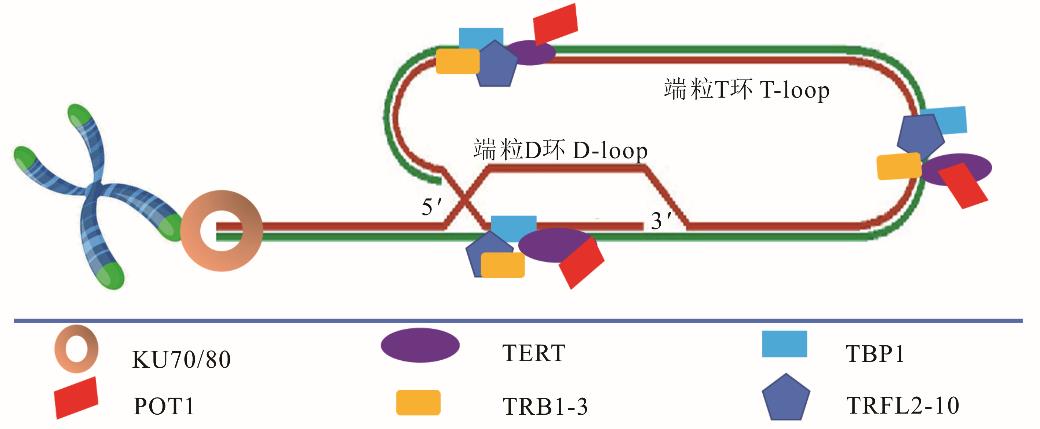
图1 端粒和端粒酶的结构KU70/80: DNA修复蛋白DNA repair protein; TERT: 端粒酶逆转录酶Telomerase reverse transcriptase; TBP1: TATA结合蛋白TATA binding protein; POT1: 端粒保护蛋白Telomere protection protein; TRB1-3: 端粒结合因子Telomere binding factor; TRFL2-10: 端粒重复序列结合因子Telomere sequence binding factor.
Fig.1 Structure of telomeres and telomerase
| 结构域Domain | 蛋白Protein | 物种Species |
|---|---|---|
含有Myb结构域的蛋白 Proteins containing the Myb domain | AtTRB1-3 | 拟南芥A.thaliana |
| AtTBP1 | 拟南芥A.thaliana | |
| AtTRFL2-10 | 拟南芥A.thaliana | |
CST 复合体蛋白 CST complex protein | AtStn1 | 拟南芥A.thaliana |
| AtTEN1 | 拟南芥A.thaliana | |
| AtCTC1 | 拟南芥A.thaliana |
表1 端粒双链DNA结合蛋白
Table 1 Telomere double-stranded DNA binding protein
| 结构域Domain | 蛋白Protein | 物种Species |
|---|---|---|
含有Myb结构域的蛋白 Proteins containing the Myb domain | AtTRB1-3 | 拟南芥A.thaliana |
| AtTBP1 | 拟南芥A.thaliana | |
| AtTRFL2-10 | 拟南芥A.thaliana | |
CST 复合体蛋白 CST complex protein | AtStn1 | 拟南芥A.thaliana |
| AtTEN1 | 拟南芥A.thaliana | |
| AtCTC1 | 拟南芥A.thaliana |
蛋白 Protein | 物种 Species | DNA结合序列 DNA binding sequence | 调控端粒酶活性 Modulate telomerase activity | 结构域 Domain | 调控端粒长度 Modulate telomere length |
|---|---|---|---|---|---|
| POT1a | 拟南芥A.thaliana | 未知Unknown | 正向Positive | OB | 延长Extension |
| POT1b | 拟南芥A.thaliana | 未知Unknown | 反向Negative | OB | 缩短Shorten |
| POT1c | 拟南芥A.thaliana | 未知Unknown | 未知Unknown | OB | 未知Unknown |
| POT1 | 小立碗藓Physcomitrella patens | GTTTAGGGTTTAGGGT | 无调控Unaffected | OB | 延长Extension |
| POT1 | 油松Pinus tabuliformis | TTAGGGTTT | 未知Unknown | OB | 未知Unknown |
| STEP1 | 拟南芥A.thaliana | (TTTAGGG)3 | 反向Negative | RBD | 缩短Shorten |
| WHY1 | 拟南芥A.thaliana | (TTTAGGG)4 | 反向Negative | Whirly | 缩短Shorten |
| WHY1 | 马铃薯Solanum tuberosum | (TTTAGGG)4 | 反向Negative | Whirly | 缩短Shorten |
| GTBP | 烟草N.tabacum | (TTTAGGG)3 | 未知Unknown | RRM | 未知Unknown |
表2 端粒单链DNA结合蛋白
Table 2 Telomere single-stranded DNA-binding proteins
蛋白 Protein | 物种 Species | DNA结合序列 DNA binding sequence | 调控端粒酶活性 Modulate telomerase activity | 结构域 Domain | 调控端粒长度 Modulate telomere length |
|---|---|---|---|---|---|
| POT1a | 拟南芥A.thaliana | 未知Unknown | 正向Positive | OB | 延长Extension |
| POT1b | 拟南芥A.thaliana | 未知Unknown | 反向Negative | OB | 缩短Shorten |
| POT1c | 拟南芥A.thaliana | 未知Unknown | 未知Unknown | OB | 未知Unknown |
| POT1 | 小立碗藓Physcomitrella patens | GTTTAGGGTTTAGGGT | 无调控Unaffected | OB | 延长Extension |
| POT1 | 油松Pinus tabuliformis | TTAGGGTTT | 未知Unknown | OB | 未知Unknown |
| STEP1 | 拟南芥A.thaliana | (TTTAGGG)3 | 反向Negative | RBD | 缩短Shorten |
| WHY1 | 拟南芥A.thaliana | (TTTAGGG)4 | 反向Negative | Whirly | 缩短Shorten |
| WHY1 | 马铃薯Solanum tuberosum | (TTTAGGG)4 | 反向Negative | Whirly | 缩短Shorten |
| GTBP | 烟草N.tabacum | (TTTAGGG)3 | 未知Unknown | RRM | 未知Unknown |
| 1 | Greider C W. Telomere length regulation. Annual Review of Biochemistry, 1996, 65(1): 337-365. |
| 2 | Harrington L. Making the most of a little: dosage effects in eukaryotic telomere length maintenance. Chromosome Research, 2005, 13(5): 493-504. |
| 3 | Kilian A, Kleinhofs A, Stiff C. Barley telomeres shorten during differentiation but grown in callus culture. Proceedings of the National Academy of Sciences, 1995, 92(21): 9555-9559. |
| 4 | Blasco M A. The epigenetic regulation of mammalian telomeres. Nature Reviews Genetics, 2007(8): 299-309. |
| 5 | Flanary B E, Kletetschk A G. Analysis of telomere length and telomerase activity in tree species of various lifespans, and with age in the bristlecone pine Pinus longaeva. Biogerontology, 2005, 6(2): 101-111. |
| 6 | Liu D, Song H, Li F L, et al. Advances in telomeres and telomerase in plants. Journal of Beijing Forestry University, 2010, 32(5): 163-167. |
| 刘頔, 宋涵, 李凤兰, 等. 植物端粒与端粒酶研究进展. 北京林业大学学报, 2010, 32(5): 163-167. | |
| 7 | Bucholc M, Buchowicz J. Synthesis of extrachromosomal DNA and telomere-related sequences in germinating wheat embryos. Seed Science Research, 1992, 2(3): 141-146. |
| 8 | Donà M, Balestrazzi A, Mondoni A, et al. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring exsitu seed longevity. Annals of Botany, 2013, 111(5): 987-998. |
| 9 | Fajkus P, Peska V, Sitova Z, et al. Allium telomeres unmasked: the unusual telomeric sequence (CTCGGTTATGGG)n is synthesized by telomerase. The Plant Journal, 2016, 85(3): 337-347. |
| 10 | Loayza D, Lange T D. POT1 as a terminal transducer of TRF1 telomere length control. Nature, 2003, 423(6943): 1013-1018. |
| 11 | Dickey T H, Altschuler S E, Wuttke D S. Single-stranded DNA-binding proteins: multiple domains for multiple functions. Structure, 2013, 21(7): 1074-1084. |
| 12 | Nugent C I, Hughes T R, Lue N F, et al. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science, 1996(272): 249-252. |
| 13 | Meng F L, Hu Y, Shen N, et al. Sua5p a single-stranded telomeric DNA-binding protein facilitates telomere replication. Embo Journal, 2014, 28(10): 1466-1478. |
| 14 | Hosseini A, Alipour A, Baradaran R V, et al. A comprehensive and mechanistic review on protective effects of kaempferol against natural and chemical toxins: Role of NF-κB inhibition and Nrf2 activation. BioFactors, 2023, 49(2): 322-350. |
| 15 | Petracek M E, Konkel L M, Kable M L, et al. A chlamydomonas protein that binds single-stranded G-strand telomere DNA. The Embo Journal, 1994, 13(15): 3648-3658. |
| 16 | Ford L P, Wrigh W E, Shay J W, et al. A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation. Oncogene, 2002(21): 580-583. |
| 17 | Dallaire F, Dupuis S, Chabot B, et al. Heterogeneous nuclear ribonucleoprotein A1 and UP1 protect mammalian telomeric repeats and modulate telomere replication in vitro. Journal of Biological Chemistry, 2000, 275(19): 14509-14516. |
| 18 | Kim M K, Kim W T. Telomere structure, function, and maintenance in plants. Journal of Plant Biology, 2018, 61: 131-136. |
| 19 | Agrawal V, Radha K K V. OB-fold: Growing bigger with functional consistency. Current Protein and Peptide Science, 2003, 4(3): 195-206. |
| 20 | Pandita T K. Critical role of the POT1 OB domain in maintaining genomic stability. Oncogene, 2016(36): 1908-1910. |
| 21 | Podell E R, Cech T R, Lei M. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nature Structural & Molecular Biology, 2004, 11(12): 1223-1229. |
| 22 | Nandakumar J, Podell E R, Lange C. How telomeric protein POT1 avoids RNA to achieve specificity for single-stranded DNA. Proceedings of the National Academy of Sciences, 2010, 107(2): 651-656. |
| 23 | Amit A, Beilstein M A, Shippen D E. Evolution of Arabidopsis protection of telomeres 1 alters nucleic acid recognition and telomerase regulation. Nucleic Acids Research, 2016, 44(20): 9821-9830. |
| 24 | Luo M, Teng X, Wang B, et al. Protection of telomeres 1 (POT1) of Pinus tabuliformis bound the telomere ssDNA. Tree Physiology, 2019, 40(1): 119-127. |
| 25 | Pennock E, Buckley K, Lundblad V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell, 2001, 104(3): 387-396. |
| 26 | Paeschke K, Juranek S, Simonsson T, et al. Telomerase recruitment by the telomere end binding protein-b facilitates g-quadruplex DNA unfolding in ciliates articles. Nature Structural & Molecular Biology, 2008, 15(6): 598-604. |
| 27 | Baumann P. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science, 2001, 292(5519): 1171-1175. |
| 28 | Hosokawa K, MacArthur B D, Ikushima Y M, et al. The telomere binding protein Pot1 maintains haematopoietic stem cell activity with age. Nature Communications, 2017(8): 804. |
| 29 | Savage S A. Human telomeres and telomere biology disorders. Progress in Molecular Biology and Translational Science, 2014(125): 41-66. |
| 30 | Shakirov E V, Song X, Joseph J A, et al. POT1 proteins in green algae and land plants: DNA-binding properties and evidence of co-evolution with telomeric DNA. Nucleic Acids Research, 2009, 37(22): 7455-7467. |
| 31 | Shakirov E V, Perroud P F, Nelson A D, et al. Protection of telomeres 1 is required for telomere integrity in the moss Physcomitrella patens. Plant Cell, 2010, 22(6): 1838-1848. |
| 32 | Desveaux D, Subramaniam R, Després C, et al. A “Whirly” transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Developmental Cell, 2004, 6(2): 229-240. |
| 33 | Yoo H H, Kwon C, Lee M M, et al. Single-stranded DNA binding factor AtWHY1 modulates telomere length homeostasis in Arabidopsis. Plant Journal, 2007(49): 442-451. |
| 34 | Janack B, Sosoi P, Kruinska K, et al. Knockdown of WHIRLY1 affects drought stress-induced leaf senescence and histone modifications of the senescence associated gene HvS40. Plants, 2016, 5(3): 37. |
| 35 | Desveaux D, Maréchal A, Brisson N. Whirly transcription factors: defense gene regulation and beyond. Trends in Plant Science, 2005, 10(2): 95-102. |
| 36 | Miao Y, Jiang J, Zhao R Z. The single-stranded DNA-binding protein WHIRLY1 represses WRKY53 expression and delays leaf senescence in a developmental stage-dependent manner in Arabidopsis. Plant Physiology, 2013, 163(2): 746-756. |
| 37 | Desveaux D, Allard J, Brisson N, et al. A new family of plant transcription factors displays a novel ssDNA-binding surface. Nature Structural Biology, 2002, 9(7): 512-517. |
| 38 | Charles D, Subramaniam R, Brisson M N. The activation of the potato PR-10a gene requires the phosphorylation of the nuclear factor PBF-1. The Plant Cell, 1995, 7(5): 589-598. |
| 39 | Subramaniam R, Charles D, Normand B. A functional homolog of mammalian protein kinase C participates in the elicitor-induced defense response in potato. The Plant Cell, 1997, 9(4): 653-664. |
| 40 | Kwon C, Chung I K. Interaction of an Arabidopsis RNA-binding protein with plant single-stranded telomeric DNA modulates telomerase activity. Journal of Biological Chemistry, 2004, 279(13): 12812-12818. |
| 41 | Yoo H H, Kwon C, Chung I K. An Arabidopsis splicing RNP variant STEP1 regulates telomere length homeostasis by restricting access of nuclease and telomerase. Molecules & Cells, 2010, 30(3): 279-283. |
| 42 | Ding J, Hayashi M K, Zhang Y, et al. Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes & Development, 1999, 13(9): 1102-1115. |
| 43 | Yong W L, Kim W T. Tobacco GTBP1, a homolog of human heterogeneous nuclear ribonucleoprotein, protects telomeres from aberrant homologous recombination. Plant Cell, 2010, 22(8): 2781-2795. |
| 44 | Hirata Y, Suzuki C, Sakai S. Characterization and gene cloning of telomere-binding protein from tobacco BY-2 cells. Plant Physiology & Biochemistry, 2004, 42(1): 7-14. |
| 45 | Lee Y W, Kim W T. Telomerase-dependent 3′ G-strand overhang maintenance facilitates GTBP1-mediated telomere protection from misplaced homologous recombination. Plant Cell, 2013(4): 1329-1342. |
| 46 | Kwon C, Kwon K, Chung I K, et al. Characterization of single stranded telomeric DNA-binding proteins in cultured soybean (Glycine max) cells. Molecules & Cells, 2004, 17(3): 503-508. |
| 47 | Kim J H, Kim W T, Chung I K. Rice proteins that bind single-stranded G-rich telomere DNA. Plant Molecular Biology, 1998(36): 661-672. |
| 48 | Lee J H, Kim J H, Kim W T, et al. Characterization and developmental expression of single-stranded telomeric DNA-binding proteins from mung bean (Vigna radiata). Plant Molecular Biology, 2000, 42(4): 547-557. |
| 49 | Croy J E, Wuttke D S. Themes in ssDNA recognition by telomere-end protection proteins. Trends in Biochemical Sciences, 2006, 31(9): 516-525. |
| 50 | Aramburu T, Kelich J, Rice C, et al. POT1-TPP1 binding stabilizes POT1, promoting efficient telomere maintenance. Computational and Structural Biotechnology Journal, 2022, 20: 675-684. |
| 51 | Zimmer A, Lang D, Richardt S, et al. Dating the early evolution of plants: detection and molecular clock analyses of orthologs. Molecular Genetics & Genomics, 2007, 278(4): 393-402. |
| 52 | Shakirov E V, Surovtseva Y V, Osbun N, et al. The Arabidopsis Pot1 and Pot2 proteins function in telomere length homeostasis and chromosome end protection. Molecular & Cellular Biology, 2005, 25(17): 7725. |
| 53 | Beilstein M A, Renfrew K B, Song X, et al. Evolution of the telomere-associated protein Pot1a in Arabidopsis thaliana is characterized by positive selection to reinforce protein-protein interaction. Molecular Biology & Evolution, 2015(5): 1329-1341. |
| 54 | Horvath M P, Schweiker V L, Bevilacqua J M, et al. Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell, 1998, 95(7): 963-974. |
| 55 | Peersen O B, Ruggles J A, Schultz S C, et al. Dimeric structure of the Oxytricha nova telomere end-binding protein α-subunit bound to ssDNA. Nature Structural Biology, 2002(9): 182-187. |
| 56 | Wojciechowski M, Fogolari F, Baginski M. Thermodynamic and electrostatic properties of ternary Oxytricha nova TEBP-DNA complex. Journal of Structural Biology, 2005, 152(3): 169-184. |
| 57 | Glustroma L W, Lyona K R, Paschinib M, et al. Single-stranded telomere-binding protein employs a dual rheostat for binding affinity and specificity that drives function. Proceedings of the National Academy of Sciences, 2018, 115(41): 10315-10320. |
| 58 | Horvath M P. Structural anatomy of telomere OB proteins. Critical Reviews in Biochemistry and Molecular Biology, 2011(46): 409-435. |
| 59 | Ay N, Irmler K, Fischer A, et al. Epigenetic programming via histone methylation at WRKY53 controls leaf senescence in Arabidopsis thaliana. Plant Journal for Cell & Molecular Biology, 2010, 58(2): 333-346. |
| 60 | Chiodi I, Mondello C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Frontiers in Oncology, 2012, 2: 133. |
| [1] | 孙守江, 毛培胜, 豆丽茹, 贾志程, 孙铭, 马馼, 欧成明, 王娟. 活性氧及染色体端粒调控种子老化研究[J]. 草业学报, 2023, 32(8): 202-213. |
| [2] | 孙守江, 师尚礼, 吴召林, 何丽娟, 金鑫, 祁娟. 激动素对盐胁迫下老芒麦幼苗端粒酶活性及生理特性的影响[J]. 草业学报, 2018, 27(11): 87-94. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||