

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2021, Vol. 30 ›› Issue (7): 82-92.DOI: 10.11686/cyxb2021016
收稿日期:2021-01-14
修回日期:2021-03-04
出版日期:2021-07-20
发布日期:2021-06-03
通讯作者:
于卓
作者简介:Corresponding author. E-mail: yuzhuo58@sina.com基金资助:
Guo-fang WU( ), Xiao-xia YU, Zhuo YU(
), Xiao-xia YU, Zhuo YU( ), Dong-sheng YANG, Qian-qian LU
), Dong-sheng YANG, Qian-qian LU
Received:2021-01-14
Revised:2021-03-04
Online:2021-07-20
Published:2021-06-03
Contact:
Zhuo YU
摘要:
采用BSA?SSR技术对高丹草低氢氰酸含量性状DNA目的片段进行了筛选和鉴定。结果表明,利用筛选出的9对SSR适宜引物PCR扩增找到了与高丹草低氰酸性状相关的SSR目的片段26个,经对PCR产物回收、部分纯化及测序分析,得到了这些低氰DNA片段的碱基序列。经与BLASTn数据库序列比对发现,有2个低氰目的片段TF8和TF16分别与高粱叶绿体磷酸核糖激酶XM_021458168.1和高粱克隆BAC 88M4 AY661656.1基因同源性高达95.4%和97.0%,其片段大小分别为101和586 bp。但在DNA碱基上有一定变异,TF8片段发生了3次碱基替换和1次插入,导致丙氨酸和甘氨酸替换成半胱氨酸,苏氨酸替换成丙氨酸,甘氨酸缺失;TF16片段缺失了1个碱基A,导致丙氨酸缺失。据此推测TF8和TF16这2个低氰目的片段具有调控低氢氰酸含量性状的作用。该研究结果为深入开展高丹草低氢氰酸含量性状主效QTL精细定位及标记辅助育种等研究奠定了基础。
吴国芳, 于肖夏, 于卓, 杨东升, 卢倩倩. 基于BSA-SSR技术的高丹草低氢氰酸性状目的片段的筛选与鉴定[J]. 草业学报, 2021, 30(7): 82-92.
Guo-fang WU, Xiao-xia YU, Zhuo YU, Dong-sheng YANG, Qian-qian LU. Screening and identification of target fragments with low cyanide traits of sorghum-sudangrass hybrid using BSA-SSR[J]. Acta Prataculturae Sinica, 2021, 30(7): 82-92.

图1 高丹草F2部分单株基因组DNA电泳检测M: DNA标记DNA marker; S: 母本散穗高粱Scattered ear sorghum (♀); R: 父本红壳苏丹草Red hull sudangrass (♂); 1~20: 部分单株Some individuals. 下同The same below.
Fig.1 Sorghum-sudangrass hybrids of F2 plants genomic DNA electrophoresis results
| 引物名称Primers | 上游引物Forward primer (5′?3′) | 下游引物Reverse primer (5′?3′) |
|---|---|---|
| AH99 | CGCACCATTCCGTTCTTG | CCGACTGTGACGCACTTGAT |
| B4195 | ACGACCACCGTCTCCAAC | CGCCTTCACCTGCTCATA |
| S64 | YTTGCGACTAGCAAAGTGG | CGAACTCCTTGTACAGGATGG |
| Xtxp7 | ACATCTACTACCCTCTCACC | ACACATCGAGACCAGTTG |
| Xtxp21 | GAGCTGCCATAGATTTGGTCG | ACCTCGACCCACCTTTGTTG |
| Xtxp31 | ACCCAAAGCCCAAATCAG | GGGGGAGAAACGGTGAG |
| Xtxp67 | CCTGACGCTCGTGGCTACC | TCCACACAAGATTCAGGCTCC |
| Xtxp183 | AAGTTGTAATGGGGCTATTG | TTAAGAGGTGGGATATTGGT |
| Xtxp321 | TAACCCAAGCCTGAGCATAAGA | CCCATTCACATGAGACGAG |
表1 9对SSR适宜引物的碱基序列
Table 1 The nucleotide sequences of 9 pairs of SSR primers combinations
| 引物名称Primers | 上游引物Forward primer (5′?3′) | 下游引物Reverse primer (5′?3′) |
|---|---|---|
| AH99 | CGCACCATTCCGTTCTTG | CCGACTGTGACGCACTTGAT |
| B4195 | ACGACCACCGTCTCCAAC | CGCCTTCACCTGCTCATA |
| S64 | YTTGCGACTAGCAAAGTGG | CGAACTCCTTGTACAGGATGG |
| Xtxp7 | ACATCTACTACCCTCTCACC | ACACATCGAGACCAGTTG |
| Xtxp21 | GAGCTGCCATAGATTTGGTCG | ACCTCGACCCACCTTTGTTG |
| Xtxp31 | ACCCAAAGCCCAAATCAG | GGGGGAGAAACGGTGAG |
| Xtxp67 | CCTGACGCTCGTGGCTACC | TCCACACAAGATTCAGGCTCC |
| Xtxp183 | AAGTTGTAATGGGGCTATTG | TTAAGAGGTGGGATATTGGT |
| Xtxp321 | TAACCCAAGCCTGAGCATAAGA | CCCATTCACATGAGACGAG |
目的片段名称 Name of target fragment | 引物名 Primer | 片段大小 Size of fragment (bp) | 目的片段名称 Name of target fragment | 引物名 Primer | 片段大小 Size of fragment (bp) |
|---|---|---|---|---|---|
| TF1 | Xtxp7 | 300 | TF14 | Xtxp321 | 750 |
| TF2 | Xtxp7 | 280 | TF15 | Xtxp321 | 600 |
| TF3 | Xtxp7 | 240 | TF16 | Xtxp321 | 550 |
| TF4 | Xtxp31 | 600 | TF17 | S64 | 750 |
| TF5 | Xtxp31 | 350 | TF18 | S64 | 500 |
| TF6 | B4195 | 500 | TF19 | S64 | 600 |
| TF7 | B4195 | 300 | TF20 | S64 | 650 |
| TF8 | B4195 | 100 | TF21 | S64 | 420 |
| TF9 | Xtxp183 | 350 | TF22 | S64 | 400 |
| TF10 | Xtxp183 | 300 | TF23 | S64 | 450 |
| TF11 | Xtxp67 | 700 | TF24 | S64 | 350 |
| TF12 | Xtxp67 | 500 | TF25 | AH99 | 150 |
| TF13 | Xtxp67 | 300 | TF26 | Xtxp21 | 180 |
表2 高丹草SSR低氰目的片段筛选
Table 2 Screening results of low cyanide target fragments in sorghum-sudangrass hybrid
目的片段名称 Name of target fragment | 引物名 Primer | 片段大小 Size of fragment (bp) | 目的片段名称 Name of target fragment | 引物名 Primer | 片段大小 Size of fragment (bp) |
|---|---|---|---|---|---|
| TF1 | Xtxp7 | 300 | TF14 | Xtxp321 | 750 |
| TF2 | Xtxp7 | 280 | TF15 | Xtxp321 | 600 |
| TF3 | Xtxp7 | 240 | TF16 | Xtxp321 | 550 |
| TF4 | Xtxp31 | 600 | TF17 | S64 | 750 |
| TF5 | Xtxp31 | 350 | TF18 | S64 | 500 |
| TF6 | B4195 | 500 | TF19 | S64 | 600 |
| TF7 | B4195 | 300 | TF20 | S64 | 650 |
| TF8 | B4195 | 100 | TF21 | S64 | 420 |
| TF9 | Xtxp183 | 350 | TF22 | S64 | 400 |
| TF10 | Xtxp183 | 300 | TF23 | S64 | 450 |
| TF11 | Xtxp67 | 700 | TF24 | S64 | 350 |
| TF12 | Xtxp67 | 500 | TF25 | AH99 | 150 |
| TF13 | Xtxp67 | 300 | TF26 | Xtxp21 | 180 |
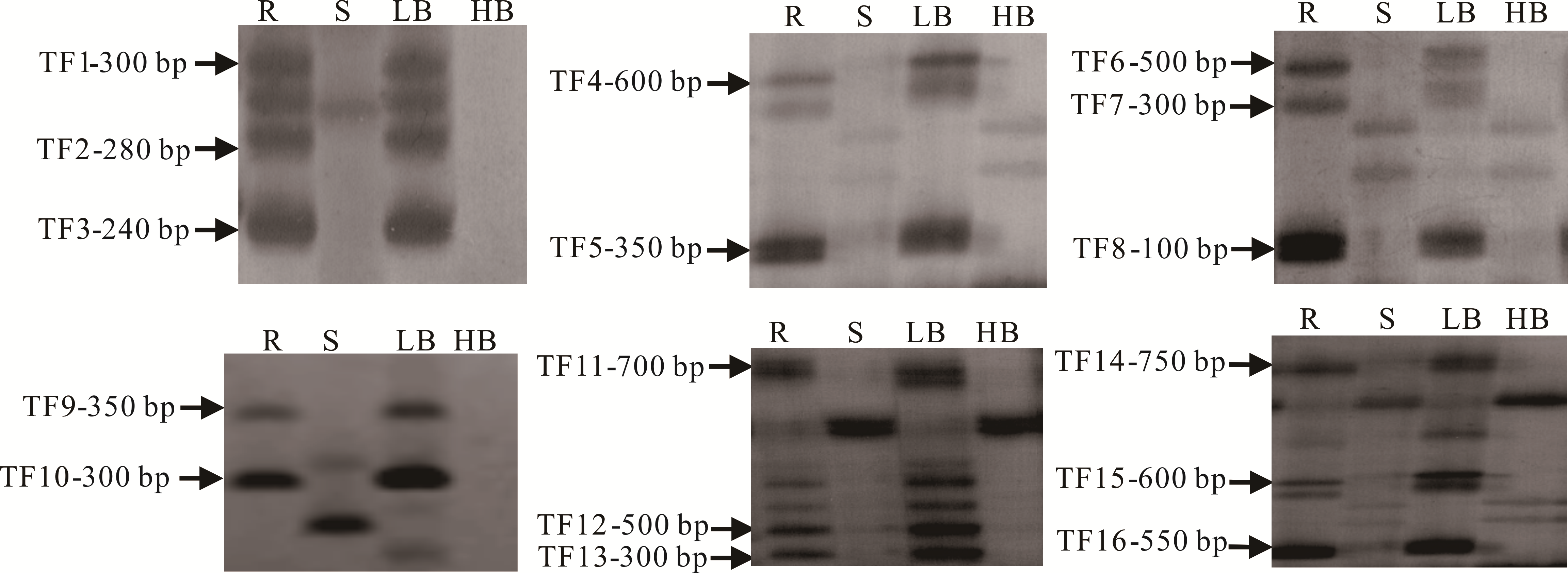
图2 高丹草BSA混池及亲本部分SSR低氰目的片段的筛选LB: 低氰基因池Low cyanide bulked gene pool; HB: 高氰基因池High cyanide bulked gene pool; TF1~TF16: 部分低氰SSR目的片段Partial low cyanide target fragments. 下同The same below.
Fig.2 Screening of low cyanide target fragments of the BSA gene pools of sorghum-sudangrass hybrid and parents

图3 26个低氰目的片段二次扩增检测a: 14个不需要纯化的低氰片段14 low cyanide fragments that do not need to be purified; b: 12个需要纯化的低氰片段12 low cyanide fragments that need to be purified; M: DNA标记DNA marker. 下同The same below.
Fig.3 Secondary amplification detection of 26 low cyanide target fragments
目的片段名称 Name of target fragment | 片段实际大小 Size of fragment (bp) | 目的片段名称 Name of target fragment | 片段实际大小 Size of fragment (bp) | 目的片段名称 Name of target fragment | 片段实际大小 Size of fragment (bp) |
|---|---|---|---|---|---|
| TF1 | 282 | TF10 | 277 | TF19 | 585 |
| TF2 | 271 | TF11 | 618 | TF20 | 589 |
| TF3 | 235 | TF12 | 447 | TF21 | 363 |
| TF4 | 451 | TF13 | 56 | TF22 | 344 |
| TF5 | 353 | TF14 | 622 | TF23 | 504 |
| TF6 | 476 | TF15 | 501 | TF24 | 297 |
| TF7 | 241 | TF16 | 586 | TF25 | 136 |
| TF8 | 101 | TF17 | 672 | TF26 | 403 |
| TF9 | 314 | TF18 | 560 |
表3 26个低氰目的片段DNA测序
Table 3 DNA sequencing results of 26 low cyanide target fragments
目的片段名称 Name of target fragment | 片段实际大小 Size of fragment (bp) | 目的片段名称 Name of target fragment | 片段实际大小 Size of fragment (bp) | 目的片段名称 Name of target fragment | 片段实际大小 Size of fragment (bp) |
|---|---|---|---|---|---|
| TF1 | 282 | TF10 | 277 | TF19 | 585 |
| TF2 | 271 | TF11 | 618 | TF20 | 589 |
| TF3 | 235 | TF12 | 447 | TF21 | 363 |
| TF4 | 451 | TF13 | 56 | TF22 | 344 |
| TF5 | 353 | TF14 | 622 | TF23 | 504 |
| TF6 | 476 | TF15 | 501 | TF24 | 297 |
| TF7 | 241 | TF16 | 586 | TF25 | 136 |
| TF8 | 101 | TF17 | 672 | TF26 | 403 |
| TF9 | 314 | TF18 | 560 |

图5 低氰目的片段TF1、TF2、TF8、TF16 的序列峰图上数字表示碱基数The figure represents the base number; a~d: 低氰目的片段TF1、TF2、TF8和TF16 TF1,TF2,TF8 and TF16 Target fragments of low cyanide.
Fig.5 Sequence peaks of some low hydrocyanic acid target fragments TF1, TF2, TF8, TF16
| 目的片段Target fragment | 长度Length (bp) | 基因 Gene | 基因描述 Gene description | 同源性Identity (%) |
|---|---|---|---|---|
| TF4 | 451 | K0J259 | 过氧化物酶受体2 Peroxisome proliferator-activated receptor gamma 2 | 100.0 |
| TF12 | 447 | A0A2K6B7D4 | 过氧化物酶受体Peroxisome proliferator-activated receptor gamma | 100.0 |
| TF26 | 403 | A0A2K6RRD2 | 过氧化物酶受体Peroxisome proliferator-activated receptor gamma | 100.0 |
| TF16 | 586 | AY661656.1 | 高粱克隆BAC 88M4基因Sorghum bicolor clone BAC 88M4 | 97.0 |
| TF17 | 672 | F8QJV4 | 干腐菌未表征蛋白Uncharacterized protein (Serpula lacrymans var. lacry…) | 96.4 |
| TF8 | 101 | XM_021458168.1 | 预测:高粱叶绿体磷酸核糖激酶Predicted: Sorghum bicolor phosphoribulokinase, chloroplastic | 95.4 |
| TF18 | 560 | MT703519.1 | 未培养真菌克隆MEL2362236_RCJ505 Uncultured fungus clone MEL2362236_RCJ505 | 91.6 |
| TF1 | 282 | XM_002758639.5 | 预测:过氧化物酶受体Predicted: peroxisome proliferator-activated receptor gamma | 88.4 |
| TF5 | 353 | AF310249.1 | 过氧化物酶受体Peroxisome proliferator-activated receptor gamma | 88.2 |
| TF21 | 363 | Q8ZQ76 | 鼠伤寒沙门氏菌氨基肽酶N Aminopeptidase N (Salmonella typhimurium stra) | 84.6 |
| TF19 | 585 | KJ173700.1 | 黄孢原毛单胞菌18S RNA基因Cladosporium flabelliforme isolate 18S RNA gene | 83.2 |
| TF20 | 589 | KJ173700.1 | 黄孢原毛单胞菌18S RNA基因Cladosporium flabelliforme isolate 18S RNA gene | 83.2 |
| TF23 | 504 | A0A072PQP8 | 磷脂酰丝氨酸脱羧酶原酶2 Phosphatidylserine decarboxylase proenzyme 2 | 81.3 |
| TF25 | 136 | A0A078FW90 | 甘蓝型油菜蛋白BnaA09g07050D protein (Brassica napus) | 81.0 |
| TF2 | 271 | ? | ? | ? |
| TF3 | 235 | ? | ? | ? |
| TF6 | 476 | ? | ? | ? |
| TF7 | 241 | ? | ? | ? |
| TF9 | 314 | ? | ? | ? |
| TF10 | 277 | ? | ? | ? |
| TF11 | 618 | ? | ? | ? |
| TF13 | 56 | ? | ? | ? |
| TF14 | 622 | ? | ? | ? |
| TF15 | 501 | ? | ? | ? |
| TF22 | 344 | ? | ? | ? |
| TF24 | 297 | ? | ? | ? |
表4 高丹草低氰目的片段同源性比对
Table 4 Identity of comparison of low cyanide target fragments in sorghum-sudangrass hybrid
| 目的片段Target fragment | 长度Length (bp) | 基因 Gene | 基因描述 Gene description | 同源性Identity (%) |
|---|---|---|---|---|
| TF4 | 451 | K0J259 | 过氧化物酶受体2 Peroxisome proliferator-activated receptor gamma 2 | 100.0 |
| TF12 | 447 | A0A2K6B7D4 | 过氧化物酶受体Peroxisome proliferator-activated receptor gamma | 100.0 |
| TF26 | 403 | A0A2K6RRD2 | 过氧化物酶受体Peroxisome proliferator-activated receptor gamma | 100.0 |
| TF16 | 586 | AY661656.1 | 高粱克隆BAC 88M4基因Sorghum bicolor clone BAC 88M4 | 97.0 |
| TF17 | 672 | F8QJV4 | 干腐菌未表征蛋白Uncharacterized protein (Serpula lacrymans var. lacry…) | 96.4 |
| TF8 | 101 | XM_021458168.1 | 预测:高粱叶绿体磷酸核糖激酶Predicted: Sorghum bicolor phosphoribulokinase, chloroplastic | 95.4 |
| TF18 | 560 | MT703519.1 | 未培养真菌克隆MEL2362236_RCJ505 Uncultured fungus clone MEL2362236_RCJ505 | 91.6 |
| TF1 | 282 | XM_002758639.5 | 预测:过氧化物酶受体Predicted: peroxisome proliferator-activated receptor gamma | 88.4 |
| TF5 | 353 | AF310249.1 | 过氧化物酶受体Peroxisome proliferator-activated receptor gamma | 88.2 |
| TF21 | 363 | Q8ZQ76 | 鼠伤寒沙门氏菌氨基肽酶N Aminopeptidase N (Salmonella typhimurium stra) | 84.6 |
| TF19 | 585 | KJ173700.1 | 黄孢原毛单胞菌18S RNA基因Cladosporium flabelliforme isolate 18S RNA gene | 83.2 |
| TF20 | 589 | KJ173700.1 | 黄孢原毛单胞菌18S RNA基因Cladosporium flabelliforme isolate 18S RNA gene | 83.2 |
| TF23 | 504 | A0A072PQP8 | 磷脂酰丝氨酸脱羧酶原酶2 Phosphatidylserine decarboxylase proenzyme 2 | 81.3 |
| TF25 | 136 | A0A078FW90 | 甘蓝型油菜蛋白BnaA09g07050D protein (Brassica napus) | 81.0 |
| TF2 | 271 | ? | ? | ? |
| TF3 | 235 | ? | ? | ? |
| TF6 | 476 | ? | ? | ? |
| TF7 | 241 | ? | ? | ? |
| TF9 | 314 | ? | ? | ? |
| TF10 | 277 | ? | ? | ? |
| TF11 | 618 | ? | ? | ? |
| TF13 | 56 | ? | ? | ? |
| TF14 | 622 | ? | ? | ? |
| TF15 | 501 | ? | ? | ? |
| TF22 | 344 | ? | ? | ? |
| TF24 | 297 | ? | ? | ? |

图7 低氰目的片段TF8和TF16碱基序列特征“?”表示碱基或氨基酸缺失。 “?” indicates the missing of bases and amino acids. 下同 The same below.
Fig.7 Base sequence characteristics of TF8 and TF16 of low cyanide target fragments
| 低氰片段Low cyanide fragment | 登录号 Login ID | 基因名称 Gene name | 编码氨基酸 Encode amino acid | 高粱BTx623 Sorghum BTx623 | 红壳苏丹草 Red hull sudangrass | 低氰F2植株 Low cyanide of F2 plant |
|---|---|---|---|---|---|---|
| TF8 | lcl|Query_51852 | XM_021458168.1 | 3个替换3 replacements | 丙氨酸Alanine、甘氨酸Glycine | 半胱氨酸Cysteine | 半胱氨酸Cysteine |
| 苏氨酸Threonine | 丙氨酸Alanine | 丙氨酸Alanine | ||||
| 1个缺失1 missing | 甘氨酸Glycine | - | - | |||
| TF16 | lcl|Query_10405 | AY661656.1 | 1个缺失1 missing | 丙氨酸Alanine | - | - |
表5 低氰片段TF8和TF16氨基酸差异
Table 5 The difference amino acids of low cyanide fragments TF8 and TF16
| 低氰片段Low cyanide fragment | 登录号 Login ID | 基因名称 Gene name | 编码氨基酸 Encode amino acid | 高粱BTx623 Sorghum BTx623 | 红壳苏丹草 Red hull sudangrass | 低氰F2植株 Low cyanide of F2 plant |
|---|---|---|---|---|---|---|
| TF8 | lcl|Query_51852 | XM_021458168.1 | 3个替换3 replacements | 丙氨酸Alanine、甘氨酸Glycine | 半胱氨酸Cysteine | 半胱氨酸Cysteine |
| 苏氨酸Threonine | 丙氨酸Alanine | 丙氨酸Alanine | ||||
| 1个缺失1 missing | 甘氨酸Glycine | - | - | |||
| TF16 | lcl|Query_10405 | AY661656.1 | 1个缺失1 missing | 丙氨酸Alanine | - | - |
| 1 | Zhan Q W, Qian Z Q. Heterosis utilization of hybrid between sorghum [Sorghum bicolor (L.) Moench] and sudangrass [Sorghum sudanense (Piper) StaTF]. Acta Agronomica Sinica, 2004, 30(1): 73-77. |
| 詹秋文, 钱章强. 高粱与苏丹草杂种优势利用的研究. 作物学报, 2004, 30(1): 73-77. | |
| 2 | Yu X X, Liu Z H, Yu Z, et al. Development of SSR markers linked to low hydrocyanic acid content in sorghum-sudan grass hybrid based on BSA method. Protein and Peptide Letters, 2016, 23(5): 417-423. |
| 3 | Hayes C M, Weers B D, Manish T, et al. Discovery of a dhurrin QTL in sorghum bicolor: Co-localization of dhurrin biosynthesis and a novel stay-green QTL. Crop Science, 2016, 56(1): 104-112. |
| 4 | Mullet J E, McCormick D, Morishige R, et al. Energy sorghum-A genetic model for the design of C4 grass bioenergy crops. JournaL of Experimental Botany, 2014, 65(13): 3479-3489. |
| 5 | Rooney W L. Sorghum improvement-integrating traditional and new technology to produce improved genotypes. Advances in Agronomy, 2004, 83: 37-109. |
| 6 | Rooney W L, Blumenthal J, Bean B, et al. Designing sorghum as a dedicated bioenergy feedstock. Biofuels, Bioproducts & Biorefining, 2010, 1(2): 147-157. |
| 7 | Wu G F, Yu Z, Lu Q Q, et al. QTL mapping of hydrocyanic acid contention in sorghum-sudangrass. Acta Botanica Boreali-Occidentalia Sinica, 2019, 39(12): 2170-2178. |
| 吴国芳, 于卓, 卢倩倩, 等. 高丹草氢氰酸含量性状的QTL定位分析. 西北植物学报, 2019, 39(12): 2170-2178. | |
| 8 | Fu L P, Liu L, Chen W, et al. The research progress of cyanogenic glucosides (CNglcs) in forage crops. Journal of Grassland and Forage Science, 2020, 41(2): 4-10. |
| 付丽平, 刘璐, 陈旺, 等. 牧草作物中氰化物的研究进展. 草学, 2020, 41(2): 4-10. | |
| 9 | Guleria G J, Kumar N. Production efficiency, forage yield, nutrient uptake and quality of sorghum sudan grass hybrid (Sorghum bicolor×Sorghum sudanense)+cowpea (Vigna unguiculata) intercropping system as influenced by sowing methods and varying seed rates of cowpea. Indian Journal of Agronomy, 2018, 63(2): 150-156. |
| 10 | Abou-Elwafa S F, Amin A E A Z, Shehzad T. Genetic mapping and transcriptional profiling of phytoremediation and heavy metals responsive genes in sorghum. Ecotoxicology and Environmental Safety, 2019, 173: 366-372. |
| 11 | Shi Y, Yu X X, Nan Z B, et al. Construction of genetic linkage map of sorghum-sudangrass based on SRAP and SSR molecular markers. Chinese Journal of Grassland, 2018, 40(5): 11-17. |
| 石悦, 于肖夏, 南志标, 等. 高丹草SRAP和SSR分子遗传连锁图谱的构建. 中国草地学报, 2018, 40(5): 11-17. | |
| 12 | Shi Y. Construction of high-density genetic linkage map and QTL identification for traits such as hydrocyanic acid content of sorghum-sudangrass. Hohhot: Inner Mongolia Agricultural University, 2018. |
| 石悦. 高丹草高密度遗传连锁图谱构建及氢氰酸含量等性状的QTL定位. 呼和浩特: 内蒙古农业大学, 2018. | |
| 13 | Belalia N, Lupini A, Djemel A, et al. Analysis of genetic diversity and population structure in Saharan maize (Zea mays L.) populations using phenotypic traits and SSR markers. Genetic Resources and Crop Evolution, 2019, 66(1): 243-257. |
| 14 | Guo Y W, Wu Y Q, Anderson J A, et al. SSR marker development, linkage mapping, and QTL analysis for establishment rate in common Bermudagrass. The Plant Genome, 2017, 10(1): 1-11. |
| 15 | Michelmore R W, Paran I, Kesseli R V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proceedings of the National Academy of Sciences of the United States of America, 1991, 88(21): 9828-9832. |
| 16 | Lv X L, Zheng K Z, Li Y, et al. Identification of major QTL for maize gray leaf spot resistance by BSA method. Journal of Maize Sciences, 2015, 23(5): 16-20. |
| 吕香玲, 郑克志, 李元, 等. 利用BSA法发掘玉米抗灰斑病主效QTL. 玉米科学, 2015, 23(5): 16-20. | |
| 17 | Rahman M S, Linsell K J, Taylor J D, et al. Fine mapping of root lesion nematode (Pratylenchus thornei) resistance loci on chromosomes 6D and 2B of wheat. Theoretical and Applied Genetics, 2020, 133(2): 635-652. |
| 18 | Zuki Z M, Rafii M Y, Ramli A, et al. Segregation analysis for bacterial leaf blight disease resistance genes in rice ‘MR219’ using SSR marker. Chilean Journal of Agricultural Research, 2020, 80(2): 227-233. |
| 19 | Shan H L, Li W F, Huang Y K, et al. Screening of polymorphic SSR molecular markers between resistant and susceptible parents for localization of brown rust resistance gene. Sugar Tech, 2020, 22(1): 1-7. |
| 20 | Han L J, Chen J, Mace E S, et al. Fine mapping of qGW1, a major QTL for grain weight in sorghum. Theoretical and Applied Genetics, 2015, 128(9): 1813-1825. |
| 21 | Zhou Y X. Molecular marker-assisted selection and breeding of new strains of sorghum-sudangrass of super-low content of HCN. Hohhot: Inner Mongolia Agricultural University, 2010. |
| 周亚星. 超低氢氰酸高丹草新品系分子标记辅助选育研究. 呼和浩特: 内蒙古农业大学, 2010. | |
| 22 | Zhou Y X, Yu X X, Yu Z, et al. Cloning of ISSR characteristic fragments related to super low hydrocyanic acid content in sorghum-sudangrass and sequence analysis. Chinese Journal of Grassland, 2012, 34(6): 75-80. |
| 周亚星, 于肖夏, 于卓, 等. 高丹草超低氢氰酸含量ISSR特征片段的克隆及序列分析. 中国草地学报, 2012, 34(6): 75-80. | |
| 23 | Wang J F, Duan L Z, Luo Z Q. Determination of CN- content in hybrid Sudan grass. Acta Prataculturae Sinica, 2002, 11(1): 43-46. |
| 汪建飞, 段立珍, 罗自琴. 杂交苏丹草中CN-含量的测定. 草业学报, 2002, 11(1): 43-46. | |
| 24 | Li J W, Li N. Establishment of rapid silver-stained method of denaturing polyacrylamide gel of microsatellite markers. Chinese Potato Journal, 2015, 29(3): 136-140. |
| 李建武, 李宁. SSR标记变性聚丙烯酰胺凝胶快速银染方法的建立. 中国马铃薯, 2015, 29(3): 136-140. | |
| 25 | Wilson A T, Calvin M. The photosynthetic cycle. CO2 dependent transients. Journal of the American Chemical Society, 1995, 77(22): 5948-5957. |
| 26 | Collin V, Issakidis-Bourguet E, Marchand C, et al. The arabidopsis plastidial thioredoxins: New functions and new insights into specificity. Journal of Biological Chemistry, 2003, 278: 23747-23752. |
| 27 | Barajas-López J D D, Serrato A J, Cazalis R, et al. Circadian regulation of chloroplastic f and m thioredoxins through control of the cca1 transcription factor. Journal of Experimental Botany, 2011, 62(6): 2039-2051. |
| 28 | Ye Y, Fulcher Y G, Sliman D J, et al. The badc and bccp subunits of chloroplast acetyl-coa carboxylase sense the ph changes of the light-dark cycle. Journal of Biological Chemistry, 2020, 295(29): 9901-9916. |
| 29 | Liu G Y, Chen K C, Zheng G M, et al. Screening and identification of female-specific DNA fragments in Channa argus using SSR-BSA. Journal of Fisheries of China, 2011, 35(2): 170-175. |
| 刘改艳, 陈昆慈, 郑光明, 等. SSR-BSA技术对乌鳢性别差异标记的初步筛选. 水产学报, 2011, 35(2): 170-175. | |
| 30 | Zhang Y H, Zhang S B, Lin J, et al. Detection of high effect site for resistance to rice stripe virus by BSA. Acta Agriculturae Boreali-Sinica, 2014, 29(2): 85-88. |
| 张云辉, 张所兵, 林静, 等. 利用BSA法检测水稻条纹叶枯病高效应抗性位点. 华北农学报, 2014, 29(2): 85-88. | |
| 31 | Liao Y, Sun B J, Sun G W, et al. The application and key problems of bulked segregant analysis on the research of molecular marker in crop. Molecular Plant Breeding, 2009, 7(1): 162-168. |
| 廖毅, 孙保娟, 孙光闻, 等. 集群分离分析法在作物分子标记研究中的应用及问题分析. 分子植物育种, 2009, 7(1): 162-168. | |
| 32 | Ercolini D. PCR-DGGE fingerprinting: Novel strategies for detection of microbes in food. Journal Microbiol Methods, 2004, 56(3): 297-314. |
| 33 | Chen Z B, Xiang S N, Jiang Z X, et al. Analysis on causes of multi-bands in researching on microbe populations by PCR-DGGE. Microbiology China, 2010, 37(1): 147-154. |
| 陈章宝, 向少能, 江震献, 等. PCR-DGGE研究微生物种群中多条带产生原因分析. 微生物学通报, 2010, 37(1): 147-154. |
| [1] | 何振富, 贺春贵, 王国栋. 栽培方式对光敏型高丹草营养成分含量与产量的影响[J]. 草业学报, 2019, 28(9): 110-122. |
| [2] | 王亚麒, 袁玲. 甜高粱、高丹草和拉巴豆对难溶性磷的活化与吸收[J]. 草业学报, 2019, 28(10): 33-43. |
| [3] | 孙秋瑾, 吕燕燕, 韩云华, 王彦荣. 高丹草种子半透特性的研究[J]. 草业学报, 2018, 27(5): 162-169. |
| [4] | 何振富, 贺春贵, 王国栋, 葛玉彬. 种植密度对光敏型高丹草营养成分及动态变化的影响[J]. 草业学报, 2018, 27(10): 93-104. |
| [5] | 贺春贵, 何振富, 王斐. 夏播复种光敏型高丹草的养分含量与产量[J]. 草业学报, 2017, 26(7): 177-189. |
| [6] | 贺春贵, 何振富, 王斐. 光敏型高丹草复种穴播高效栽培模式研究[J]. 草业学报, 2017, 26(5): 70-80. |
| [7] | 梁欢, 刘贵波, 吴佳海, 曾兵, 李源, 游永亮, 赵海明. 混贮模式对高丹草青贮发酵品质及体外产气动力学特性的影响[J]. 草业学报, 2016, 25(4): 188-196. |
| [8] | 何振富, 贺春贵, 魏玉明, 刘陇生. 光敏型高丹草在陇东旱塬的生物学特性和营养成分比较研究[J]. 草业学报, 2015, 24(10): 166-174. |
| [9] | 于卓,谢锐,于肖夏,马艳红,李造哲,纪明妹. 低氢氰酸含量高丹草新品系及其亲本的SSR分析[J]. 草业学报, 2014, 23(1): 223-228. |
| [10] | 房永雨,于肖夏,于卓,马艳红,李晓宇,李造哲. 低氰含量高丹草新品系主要农艺特性及染色体构型分析[J]. 草业学报, 2012, 21(2): 162-170. |
| [11] | 刘建宁,石永红,王运琦,郭锐,吴欣明,郭璞,张燕,高新中. 高丹草生长动态及收割期的研究[J]. 草业学报, 2011, 20(1): 31-37. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||