

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2023, Vol. 32 ›› Issue (1): 165-177.DOI: 10.11686/cyxb2022189
• 研究论文 • 上一篇
姜凯( ), 吴雪莉(
), 吴雪莉( ), 刘奕君, 马越, 宋洋, 卢文杰, 王增裕
), 刘奕君, 马越, 宋洋, 卢文杰, 王增裕
收稿日期:2022-04-27
修回日期:2022-06-16
出版日期:2023-01-20
发布日期:2022-11-07
通讯作者:
吴雪莉
作者简介:E-mail: xueli0510qau@163.com基金资助:
Kai JIANG( ), Xue-li WU(
), Xue-li WU( ), Yi-jun LIU, Yue MA, Yang SONG, Wen-jie LU, Zeng-yu WANG
), Yi-jun LIU, Yue MA, Yang SONG, Wen-jie LU, Zeng-yu WANG
Received:2022-04-27
Revised:2022-06-16
Online:2023-01-20
Published:2022-11-07
Contact:
Xue-li WU
摘要:
海滨雀稗作为耐盐性较强的暖季型草坪草,具有极大的应用潜力。为建立高效稳定的海滨雀稗遗传转化体系,本研究以海滨雀稗成熟种子为外植体,确定了筛选剂潮霉素和草丁膦在海滨雀稗的愈伤组织继代培养和再生阶段的最佳筛选压。在进一步优化遗传转化条件的基础上,比较了以hpt与bar基因作为转基因筛选标记对海滨雀稗农杆菌介导的遗传转化效率的影响。结果表明:愈伤组织的再生率在继代36周后显著下降,继代48周的最高再生率为67.9%。潮霉素的最佳筛选压在继代培养阶段为120 mg·L-1,再生阶段为80 mg·L-1。草丁膦的最佳筛选压均为1.2 mg·L-1。选取继代24周的愈伤组织用于农杆菌介导的遗传转化,最佳的转化条件为菌液浓度OD600=0.6,添加100 μmol·L-1乙酰丁香酮和0.01%的Silwet L-77,并辅以超声波处理20 min或真空处理10 min,浸染30 min后共培养2 d。通过多次抗性愈伤组织和抗性再生苗的筛选,2种载体均获得了转基因再生植株。通过PCR检测证明hpt和bar基因分别成功在海滨雀稗基因组中表达。以潮霉素作为筛选剂,β-葡萄糖醛酸糖苷酶(GUS)基因稳定表达及PCR检测的转基因的平均效率为18.9%,最高转化效率为20.3%,均高于以草丁膦为筛选剂的转化效率。由此可见,hpt基因更适合作为海滨雀稗遗传转化中的筛选标记,能够获得更高的转基因效率。
姜凯, 吴雪莉, 刘奕君, 马越, 宋洋, 卢文杰, 王增裕. 海滨雀稗以hpt与bar基因为筛选标记的转化体系比较研究[J]. 草业学报, 2023, 32(1): 165-177.
Kai JIANG, Xue-li WU, Yi-jun LIU, Yue MA, Yang SONG, Wen-jie LU, Zeng-yu WANG. Comparative study on transformation systems of seashore paspalum using hpt and bar genes as selection markers[J]. Acta Prataculturae Sinica, 2023, 32(1): 165-177.
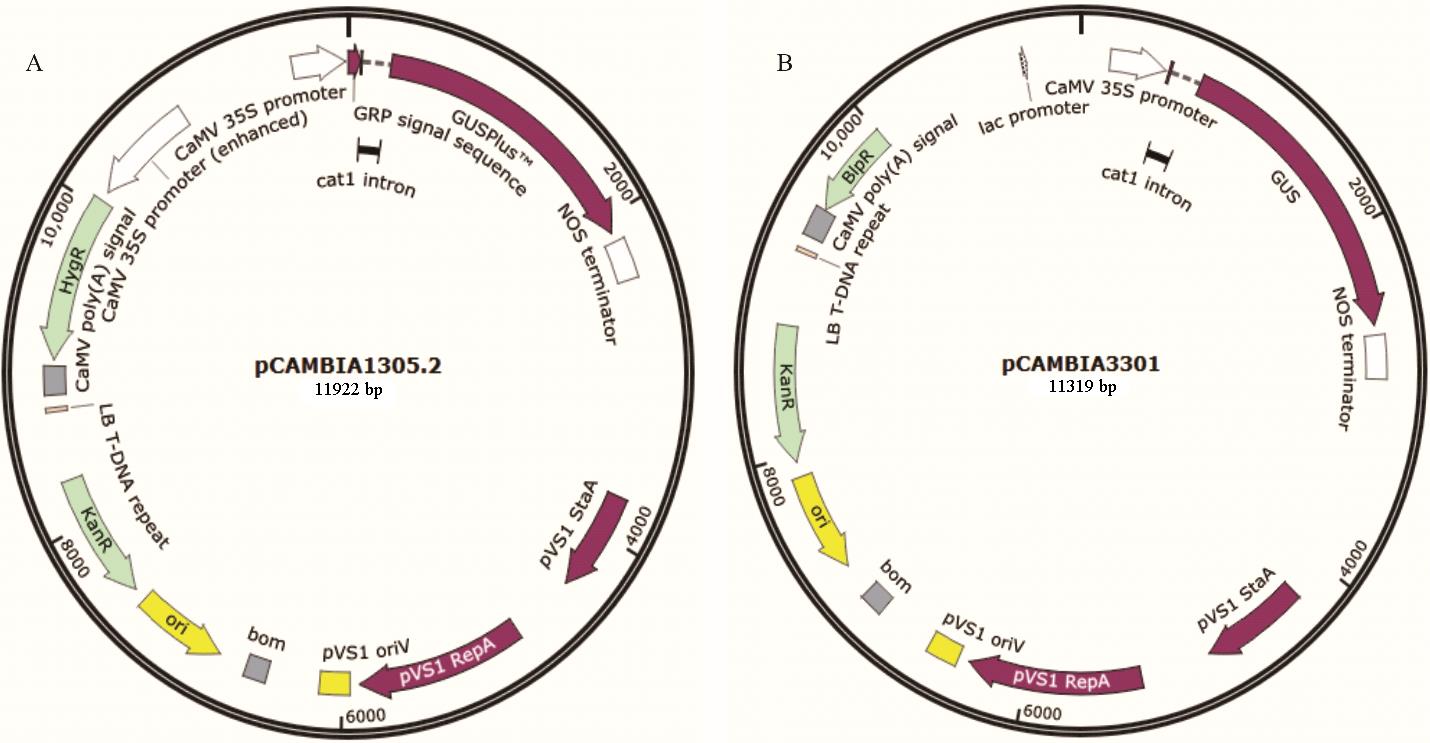
图1 植物表达载体图谱A、B分别为质粒pCAMBIA1305.2和pCAMBIA3301的结构示意图。A and B are structural representation of plasmid pCAMBIA1305.2 and pCAMBIA3301, respectively.
Fig.1 Map of plant expression vector
| 培养基类型Medium type | 培养基配方Formula of medium | 其他Others |
|---|---|---|
| 诱导和继代培养基Induction and subculture media | MS基本培养基+2.5 mg·L-1 2,4-二氯苯氧乙酸+0.6 mg·L-1 CuSO4 MS basic medium+2.5 mg·L-1 2,4-dichlorophenoxy acetic acid (2,4-D)+0.6 mg·L-1 CuSO4 | 添加30 g·L-1蔗糖,调pH值为5.8,加入8 g·L-1琼脂,121 ℃高温灭菌20 min。Added 30 g·L-1 sucrose, adjusted the pH value to 5.8, add 8 g·L-1 agar, and sterilized at 121 ℃ for 20 min. |
| 选择继代培养基Selective subculture medium | MS基本培养基+2.5 mg·L-1 2,4-D+0.6 mg·L-1 CuSO4+200 mg·L-1头孢噻肟+适宜浓度筛选剂MS basic medium+2.5 mg·L-1 2,4-D+0.6 mg·L-1 CuSO4+200 mg·L-1 cefotaxime+suitable concentration screening agent | |
| 再生培养基Regeneration medium | MS基本培养基+0.05 mg·L-1激动素+8.0 mg·L-1 6-苄氨基嘌呤+10 mg·L-1 CuSO4 MS basic medium+0.05 mg·L-1 kinetin (KT)+8.0 mg·L-1 6-benzylaminopurine (6-BA)+10 mg·L-1 CuSO4 | |
| 选择再生培养基Select regeneration medium | MS基本培养基+0.05 mg·L-1激动素+8.0 mg·L-1 6-苄氨基嘌呤+10 mg·L-1 CuSO4+200 mg·L-1头孢噻肟+适宜浓度筛选剂MS basic medium+0.05 mg·L-1 KT+8.0 mg·L-1 6-BA+10 mg·L-1 CuSO4+200 mg·L-1 cefotaxime+suitable concentration screening agent | |
| 壮苗培养基Strong seedling medium | 1/2 MS基本培养基1/2 MS basic medium | |
| 选择壮苗培养基Select seedling growth medium | 1/2 MS基本培养基+适宜浓度筛选剂1/2 MS basic medium+suitable concentration screening agent |
表1 培养基的类型和配制方法
Table 1 Types and preparation methods of culture medium
| 培养基类型Medium type | 培养基配方Formula of medium | 其他Others |
|---|---|---|
| 诱导和继代培养基Induction and subculture media | MS基本培养基+2.5 mg·L-1 2,4-二氯苯氧乙酸+0.6 mg·L-1 CuSO4 MS basic medium+2.5 mg·L-1 2,4-dichlorophenoxy acetic acid (2,4-D)+0.6 mg·L-1 CuSO4 | 添加30 g·L-1蔗糖,调pH值为5.8,加入8 g·L-1琼脂,121 ℃高温灭菌20 min。Added 30 g·L-1 sucrose, adjusted the pH value to 5.8, add 8 g·L-1 agar, and sterilized at 121 ℃ for 20 min. |
| 选择继代培养基Selective subculture medium | MS基本培养基+2.5 mg·L-1 2,4-D+0.6 mg·L-1 CuSO4+200 mg·L-1头孢噻肟+适宜浓度筛选剂MS basic medium+2.5 mg·L-1 2,4-D+0.6 mg·L-1 CuSO4+200 mg·L-1 cefotaxime+suitable concentration screening agent | |
| 再生培养基Regeneration medium | MS基本培养基+0.05 mg·L-1激动素+8.0 mg·L-1 6-苄氨基嘌呤+10 mg·L-1 CuSO4 MS basic medium+0.05 mg·L-1 kinetin (KT)+8.0 mg·L-1 6-benzylaminopurine (6-BA)+10 mg·L-1 CuSO4 | |
| 选择再生培养基Select regeneration medium | MS基本培养基+0.05 mg·L-1激动素+8.0 mg·L-1 6-苄氨基嘌呤+10 mg·L-1 CuSO4+200 mg·L-1头孢噻肟+适宜浓度筛选剂MS basic medium+0.05 mg·L-1 KT+8.0 mg·L-1 6-BA+10 mg·L-1 CuSO4+200 mg·L-1 cefotaxime+suitable concentration screening agent | |
| 壮苗培养基Strong seedling medium | 1/2 MS基本培养基1/2 MS basic medium | |
| 选择壮苗培养基Select seedling growth medium | 1/2 MS基本培养基+适宜浓度筛选剂1/2 MS basic medium+suitable concentration screening agent |

图2 愈伤组织年龄对再生的影响A:用于遗传转化的黄色颗粒状愈伤组织;B:生长12周的胚性愈伤组织;C:愈伤组织的再生;D:再生植株。不同小写字母表示差异显著(P<0.05)。下同。A: Yellow granules and firm structure callus that prepared for transformation; B: 12-week-old embryogenic callus; C: Callus regenerated; D: Regeneration plants. Different lowercase letters represented difference were significant (P<0.05). The same below.
Fig.2 Effect of callus age on regeneration
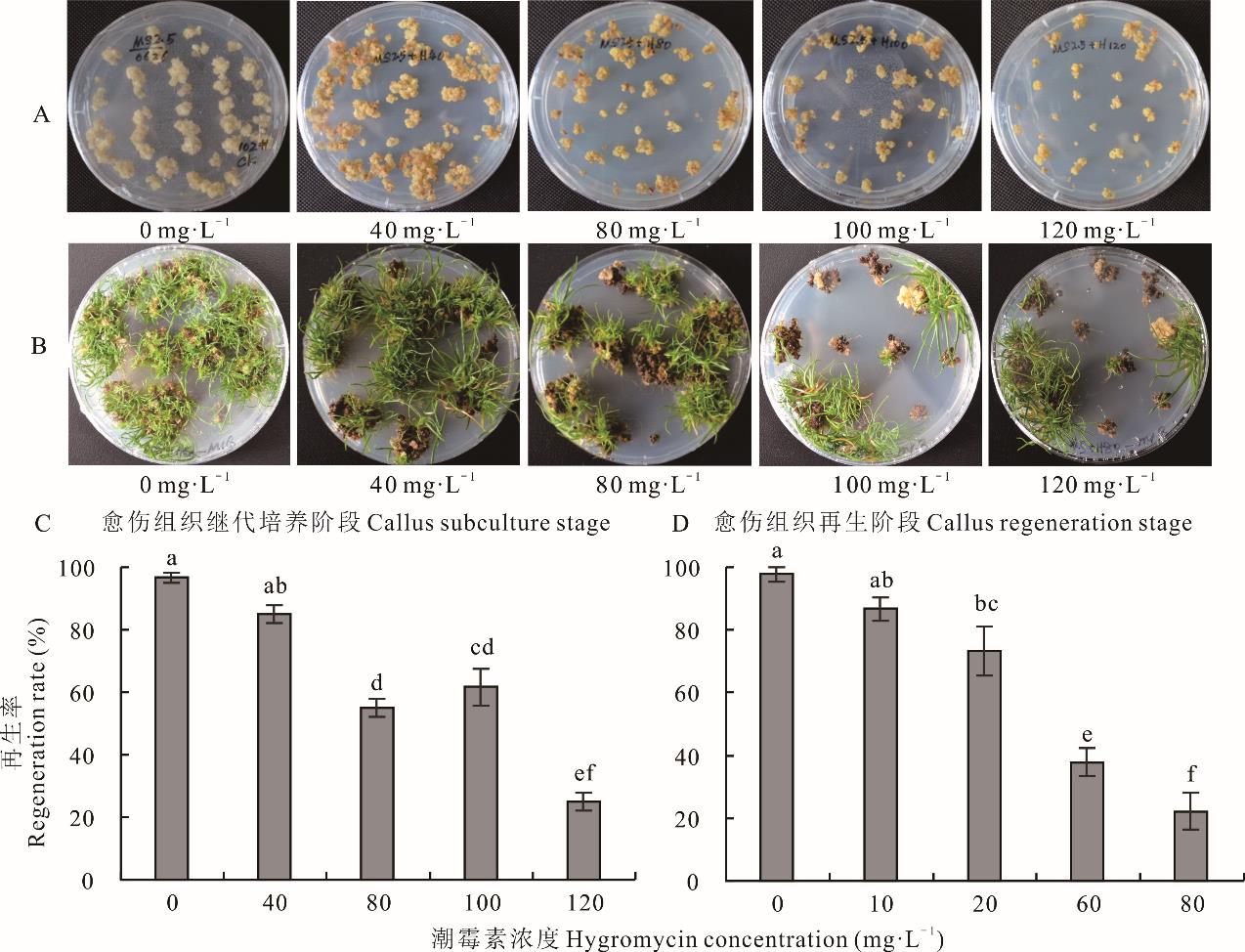
图3 潮霉素对胚性愈伤组织继代培养和再生的影响A:潮霉素浓度对愈伤组织继代培养的影响;B:潮霉素浓度对愈伤组织再生的影响。A: Effects of hygromycin concentration on callus subculture; B: Effects of hygromycin concentration on callus regeneration.
Fig.3 Effects of hygromycin on embryogenic callus subculture and regeneration
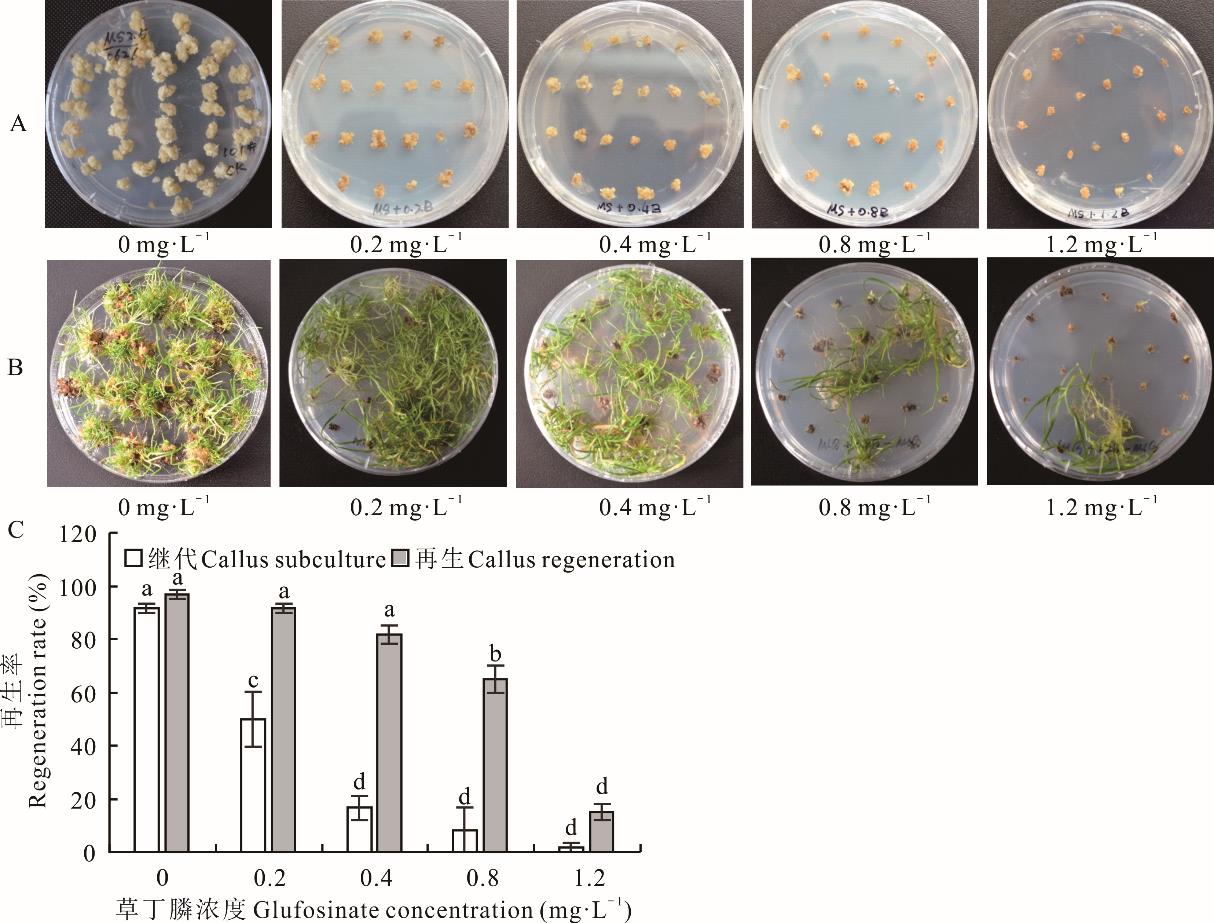
图4 草丁膦对胚性愈伤组织继代培养和再生的影响A:草丁膦浓度对愈伤组织继代培养的影响;B:草丁膦浓度对愈伤组织再生的影响。A: Effects of glufosinate concentration on callus subculture; B: Effects of glufosinate concentration on callus regeneration.
Fig.4 Effects of glufosinate on embryogenic callus subculture and regeneration

图5 不同因素对GUS瞬时表达效率的影响A~D:在添加AS、Silwet L-77、超声波处理、真空处理条件下,愈伤组织GUS瞬时表达的染色情况。A-D: Staining of GUS transient expression of callus that treated with AS, Silwet L-77, sonication or vacuum.
Fig.5 Factors affecting on percentage of transient GUS expression

图6 农杆菌介导的胚性愈伤组织的遗传转化及GUS染色A:潮霉素抗性的愈伤组织;B:GUS在潮霉素抗性愈伤组织中稳定表达;C:潮霉素抗性的再生苗;D:草丁膦抗性的愈伤组织;E:GUS在草丁膦抗性愈伤组织中稳定表达;F:草丁膦抗性的再生苗;G:抗性再生苗在温室中种植;H:GUS在抗性再生苗叶片中稳定表达。A: Hygromycin resistant calli; B: Stable expression of GUS gene in hygromycin resistant calli; C: Hygromycin resistant regenerated plants; D: Glufosinate resistant callus; E: Stable expression of GUS gene in glufosinate resistant calli; F: Glufosinate resistant regenerated plants; G: Resistant regenerated plants planted in greenhouse; H: Stable expression of GUS gene in leaves of resistant plants.
Fig.6 Agrobacterium-mediated transformation of embryogenic calli and result of GUS staining
筛选标记 Selectable marker | 接种愈伤组织数No. of infected calli | 抗性愈伤组织数No. of resistant calli | 愈伤组织GUS染色数No. of GUS (+) calli | 抗性再生植株的克隆数No. of resistant plant clones | 再生植株的GUS染色数No. of GUS (+) plant lines | 转基因效率 Transformation efficiency (%) | 平均转基因效率 Average transformation efficiency (%, mean±SD) |
|---|---|---|---|---|---|---|---|
| hpt | 63 | 10 | 10 | 6 | 6 | 9.52 | 14.46±4.69 |
| 59 | 17 | 15 | 15 | 12 | 20.34 | ||
| 49 | 10 | 7 | 7 | 6 | 12.24 | ||
| 77 | 15 | 11 | 9 | 9 | 11.69 | ||
| 81 | 22 | 22 | 18 | 15 | 18.52 | ||
| bar | 89 | 7 | 5 | 5 | 3 | 3.37 | 5.65±2.61 |
| 60 | 9 | 9 | 7 | 6 | 10.00 | ||
| 79 | 8 | 7 | 7 | 4 | 5.06 | ||
| 76 | 4 | 3 | 3 | 3 | 3.95 | ||
| 51 | 6 | 6 | 5 | 3 | 5.88 |
表2 两种不同质粒的农杆菌介导的遗传转化效率
Table 2 Summary of transformation efficiencies of Agrobacterium transformation using embryogenic callus
筛选标记 Selectable marker | 接种愈伤组织数No. of infected calli | 抗性愈伤组织数No. of resistant calli | 愈伤组织GUS染色数No. of GUS (+) calli | 抗性再生植株的克隆数No. of resistant plant clones | 再生植株的GUS染色数No. of GUS (+) plant lines | 转基因效率 Transformation efficiency (%) | 平均转基因效率 Average transformation efficiency (%, mean±SD) |
|---|---|---|---|---|---|---|---|
| hpt | 63 | 10 | 10 | 6 | 6 | 9.52 | 14.46±4.69 |
| 59 | 17 | 15 | 15 | 12 | 20.34 | ||
| 49 | 10 | 7 | 7 | 6 | 12.24 | ||
| 77 | 15 | 11 | 9 | 9 | 11.69 | ||
| 81 | 22 | 22 | 18 | 15 | 18.52 | ||
| bar | 89 | 7 | 5 | 5 | 3 | 3.37 | 5.65±2.61 |
| 60 | 9 | 9 | 7 | 6 | 10.00 | ||
| 79 | 8 | 7 | 7 | 4 | 5.06 | ||
| 76 | 4 | 3 | 3 | 3 | 3.95 | ||
| 51 | 6 | 6 | 5 | 3 | 5.88 |
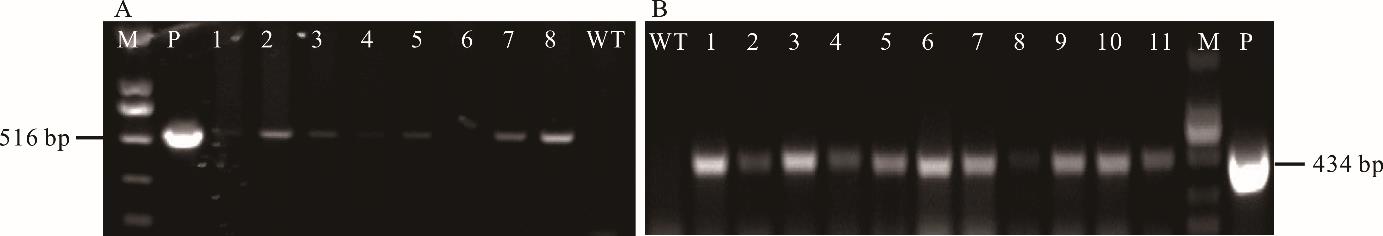
图7 抗性再生植株的PCR检测A:PCR检测抗性再生植株的hpt基因;B:PCR检测抗性再生植株的bar基因;M:标尺;P:质粒阳性对照;WT:野生型阴性对照;1~11:抗性再生植株。A: PCR detection of the hpt gene in resistant plants; B: PCR detection of the bar gene in resistant plants; M: Marker; P: Plasmid as positive control; WT: Wild type as negative control; 1-11: Resistant transgenic lines.
Fig.7 PCR test results of resistant regenerated plants
| 1 | Qu M Y, Xu H B, Chen J, et al. Molecular mechanisms and improvement of salinity tolerance in rice. Journal of Plant Genetic Resources, 2022, 23(3): 644-653. |
| 曲梦宇, 许惠滨, 陈静, 等. 水稻耐盐分子机制与分子改良. 植物遗传资源学报, 2022, 23(3): 644-653. | |
| 2 | Singh M, Singh A, Prasad S M, et al. Regulation of plants metabolism in response to salt stress: An omics approach. Acta Physiologiae Plantarum, 2017, 39(2): 48-62. |
| 3 | Shrivastava P, Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences, 2015, 22(2): 123-131. |
| 4 | Shabala S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany, 2013, 112(7): 1209-1221. |
| 5 | Himabindu Y, Chakradhar T, Reddy M C, et al. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environmental and Experimental Botany, 2016, 124: 39-63. |
| 6 | Duncan R R. Environmental compatibility of seashore paspalum (saltwater couch) for golf courses and other recreational uses I. Breeding and genetics. International Turfgrass Society Research Journal, 1999, 8(11): 1208-1215. |
| 7 | Lee G, Carrow R N, Duncan R R, et al. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environmental and Experimental Botany, 2008, 63(1/2/3): 19-27. |
| 8 | Pompeiano A, Di P E, Volterrani M, et al. Growth responses and physiological traits of seashore paspalum subjected to short-term salinity stress and recovery. Agricultural Water Management, 2016, 163: 57-65. |
| 9 | Karimi I Y M, Kurup S S, Salem M M, et al. Evaluation of bermuda and paspalum grass types for urban landscapes under saline water irrigation. Journal of Plant Nutrition, 2018, 41(7): 888-902. |
| 10 | Neibaur I, Gallo M, Altpeter F. The effect of auxin type and cytokinin concentration on callus induction and plant regeneration frequency from immature inflorescence segments of seashore paspalum (Paspalum vaginatum Swartz). In Vitro Cellular and Developmental Biology-Plant, 2008, 44(6): 480-489. |
| 11 | Liu J, Yang Z, Li W, et al. Improving cold tolerance through in vitro selection for somaclonal variations in seashore paspalum. Journal of the American Society for Horticultural Science, 2013, 138(6): 452-460. |
| 12 | Wu X, Shi H, Chen X, et al. Establishment of Agrobacterium-mediated transformation of seashore paspalum (Paspalum vaginatum O. Swartz). In Vitro Cellular & Developmental Biology-Plant, 2018, 54(5): 545-552. |
| 13 | Serena M, Schiavon M, Sallenave R, et al. Nitrogen fertilization of warm-season turfgrasses irrigated with saline water from varying irrigation systems. 1. Quality, spring green-up and fall colour retention. Journal of Agronomy and Crop Science, 2018, 204(3): 252-264. |
| 14 | Luo X B, Xiang Z X, Hu L Q. Turf quality of seashore paspalum line 09-1. Crop Research, 2013, 27(1): 57-61. |
| 罗小波, 向佐湘, 胡立群. 09-1海滨雀稗草坪坪用性状评价. 作物研究, 2013, 27(1): 57-61. | |
| 15 | Liu Z W, Zhong X X, Qian C, et al. Evaluation of growth characteristics and turf quality of new strains of self-compatible Paspalum vaginatum. Chinese Journal of Grassland, 2016, 38(6): 85-92. |
| 刘智微, 钟小仙, 钱晨, 等. 自交结实海滨雀稗新品系生长特性及坪用质量评价. 中国草地学报, 2016, 38(6): 85-92. | |
| 16 | Zhong X X, Liu Z W, Qian C, et al. Effects of sea salt stress on plant morphology and growth of a new line of seashore paspalum SP2008-3. Jiangsu Agricultural Sciences, 2016, 44(2): 285-287. |
| 钟小仙, 刘智微, 钱晨, 等. 海盐胁迫对海雀稗新品系SP2008-3植株形态与生长量的影响. 江苏农业科学, 2016, 44(2): 285-287. | |
| 17 | Liu T Z, Xie X C, Zhang J M. Mutagenic effect of 60Co-γ irradiation on turf characteristics of Paspalum vaginatum. Acta Prataculturae Sinica, 2017, 26(7): 62-70. |
| 刘天增, 谢新春, 张巨明. 海滨雀稗60Co-γ辐射诱变突变体筛选. 草业学报, 2017, 26(7): 62-70. | |
| 18 | Zhang Y, Yan J J, Bai S Q, et al. Progress of Agrobacterium-mediated genetic transformation in Gramineae. Journal of Grassland and Forage Science, 2016(4): 4-9. |
| 张瑜, 鄢家俊, 白史且, 等. 农杆菌介导禾本科植物遗传转化的研究进展. 草业与畜牧, 2016(4): 4-9. | |
| 19 | Ao T G B Y, Gao L J, Li Y Q, et al. Research progress on drought-resistant genetic engineering of gramineae forage grass. Molecular Plant Breeding, 2016, 14(11): 3079-3085. |
| 敖特根白音, 高立杰, 李运起, 等. 禾本科牧草抗旱基因工程研究进展. 分子植物育种, 2016, 14(11): 3079-3085. | |
| 20 | Wu X L, Liu J X, Nielsen K K, et al. Agrobacterium-mediated transformation of Brachypodium distachyon through embryogenic calli derived from immature embryos. Acta Prataculturae Sinica, 2010, 19(5): 9-16. |
| 吴雪莉, 刘金星, Nielsen K K, 等. 二穗短柄草幼胚再生体系及农杆菌介导转化的初步研究. 草业学报, 2010, 19(5): 9-16. | |
| 21 | Wang Y, Li J L, Pan Y N, et al. Research progress in transgenic breeding of excellent warm-season turfgrass. Grassland and Turf, 2007(4): 13-17. |
| 王艳, 李建龙, 潘永年, 等. 草坪草转基因育种研究进展. 草原与草坪, 2007(4): 13-17. | |
| 22 | Peng K Z, Liu Y Y, Lan C J, et al. Effects of different explant types and plant growth regulators on tissue culture of Gymnadenia conopsea. Modern Agricultural Science and Technology, 2021(7): 62-64. |
| 彭克忠, 刘燕云, 兰常军, 等. 不同外植体类型和植物生长调节剂对手参组培的影响. 现代农业科技, 2021(7): 62-64. | |
| 23 | Yuan B, Ding Y, Cao H Z, et al. Establishment of an efficient genetic transformation system for rice with glyphosate as a selection marker. Journal of Yunnan Agricultural University (Natural Science), 2021, 36(4): 559-565. |
| 袁冰, 丁筠, 曹含章, 等. 草甘膦为筛选标记的水稻高效遗传转化体系的建立. 云南农业大学学报(自然科学), 2021, 36(4): 559-565. | |
| 24 | Wang X C, Cheng Z Q, Zeng Q C, et al. Advances in selection marker genes of plant transformation. Journal of Anhui Agricultural Sciences, 2011, 39(22): 13290-13291, 13365. |
| 王相春, 程在全, 曾千春, 等. 植物筛选标记基因应用进展. 安徽农业科学, 2011, 39(22): 13290-13291, 13365. | |
| 25 | Liu S C, Zhang G C, Yang L F, et al. Bialaphos-resistant transgenic soybeans produced by the Agrobacterium-mediated cotyledonary-node method. Journal of Agricultural Science & Technology, 2014, 16(1): 175-190. |
| 26 | Song G Q, Walworth A, Hancock J F. Factors influencing Agrobacterium-mediated transformation of switchgrass cultivars. Plant Cell, Tissue and Organ Culture, 2012, 108(3): 445-453. |
| 27 | Hwang O J, Cho M A, Han Y J, et al. Agrobacterium-mediated genetic transformation of Miscanthus sinensis. Plant Cell, Tissue and Organ Culture, 2014, 117(1): 51-63. |
| 28 | Ran Y D, Patron N, Yu Q, et al. Agrobacterium-mediated transformation of Lolium rigidum Gaud. Plant Cell, Tissue and Organ Culture, 2014, 118(1): 67-75. |
| 29 | Liu M X, Lu S Y, Liu L, et al. Agrobacterium-mediated transformation of centipedegrass [Eremochloa ophiuroides (Munro) Hack.]. Plant Cell, Tissue and Organ Culture, 2012, 109(3): 557-563. |
| 30 | Li R, Qu R. High throughput Agrobacterium-mediated switchgrass transformation. Biomass and Bioenergy, 2011, 35(3): 1046-1054. |
| 31 | Ge Y, Norton T, Wang Z Y. Transgenic zoysiagrass (Zoysia japonica) plants obtained by Agrobacterium-mediated transformation. Plant Cell Reports, 2006, 25(8): 792-798. |
| 32 | Dong S, Qu R. High efficiency transformation of tall fescue with Agrobacterium tumefaciens. Plant Science, 2005, 168(6): 1453-1458. |
| 33 | Huang W H, Wu B Q, Yan M X. Development of Agrobacterium tumefaciens mediated transformation of Cylindrosporium eleocharidis and analysis of T-DNA insertional mutants. Southwest China Journal of Agricultural Sciences, 2020, 33(8): 1696-1702. |
| 黄伟华, 吴碧球, 颜梅新. 根癌农杆菌介导荸荠秆枯病菌转化体系构建及突变体筛选. 西南农业学报, 2020, 33(8): 1696-1702. | |
| 34 | Han W J, Zhu C R, Xu J, et al. Induction of hairy root and introduction of foreign gene in Aquilaria sinensis. Molecular Plant Breeding, 2021, 19(15): 5010-5016. |
| 韩文静, 朱楚然, 徐娇, 等. 白木香毛状根的诱导及外源基因的导入. 分子植物育种, 2021, 19(15): 5010-5016. | |
| 35 | Bidney D, Scelonge C, Martich J, et al. Microprojectile bombardment of plant tissues increases transformation frequency by Agrobacterium tumefaciens. Plant Molecular Biology, 1992, 18(2): 301-313. |
| 36 | Khan S, Fahim N, Singh P, et al. Agrobacterium tumefaciens mediated genetic transformation of Ocimum gratissimum: A medicinally important crop. Industrial Crops and Products, 2015, 71: 138-146. |
| 37 | Tiwari V, Chaturvedi A K, Mishra A, et al. An efficient method of Agrobacterium-mediated genetic transformation and regeneration in local Indian cultivar of groundnut (Arachis hypogaea) using grafting. Applied Biochemistry and Biotechnology, 2015, 175(1): 436-453. |
| 38 | Li F S, Li M Y, Zhan C, et al. A reliable and high-efficiency Agrobacterium tumefaciens-mediated transformation system of Pogonatherum paniceum embryogenic callus using GFP as a reporter gene. Plant Cell, Tissue and Organ Culture, 2015, 120(1): 155-165. |
| 39 | Huang X Q, Wei Z M. Successful Agrobacterium-mediated genetic transformation of maize elite inbred lines. Plant Cell, Tissue and Organ Culture, 2005, 83: 187-200. |
| 40 | Zhang L, Guo P Y, Zhang C H, et al. Establishment of Agrobacterium tumefaciens-mediated genetic transformation system of eGFP gene in Pericallis hybrida. Molecular Plant Breeding, 2020, 18(19): 6350-6358. |
| 张丽, 郭佩瑶, 张春华, 等. 农杆菌介导的eGFP基因转化瓜叶菊技术体系的建立. 分子植物育种, 2020, 18(19): 6350-6358. | |
| 41 | Chen L, Cong Y Y, He H X, et al. Maize (Zea mays L.) transformation by Agrobacterium tumefaciens infection of pollinated ovules. Journal of Biotechnology, 2014, 171(1): 8-16. |
| 42 | Yang A F, He C M, Zhang K W, et al. Improvement of Agrobacterium-mediated transformation of embryogenic calluses from maize elite inbred lines. In Vitro Cellular & Developmental Biology-Plant, 2006, 42(3): 215-219. |
| 43 | Sun S J, Yang S X, Feng X Z. Molecular characterization of the transgenic soybean plants by sonication-assisted Agrobacterium-mediated transformation using the embryonic tips. Soybean Science, 2014, 33(6): 808-814. |
| 孙式静, 杨素欣, 冯献忠. 超声波辅助处理农杆菌介导大豆胚尖转化转基因植株的获得和分子鉴定. 大豆科学, 2014, 33(6): 808-814. | |
| 44 | Yang L P, Wang Y, Meng D W, et al. Establishment and optimization of genetic transformation system for germinated-seed vacuum infection. Agricultural Science Journal of Yanbian University, 2019, 41(1): 58-61. |
| 杨丽萍, 王悦, 孟大伟, 等. 种子真空侵染法遗传转化体系的建立与优化. 延边大学农学学报, 2019, 41(1): 58-61. |
| [1] | 张翔, 杨勇, 刘学勇, 向佐湘. 外源水杨酸对低温胁迫下海滨雀稗抗寒生理特征的影响[J]. 草业学报, 2020, 29(1): 117-124. |
| [2] | 杨轲, 陈一酉, 汪军成, 姚立蓉, 孟亚雄, 马小乐, 李葆春, 司二静, 王化俊. 啤酒花不同外植体筛选及再生体系构建[J]. 草业学报, 2019, 28(8): 209-217. |
| [3] | 钱晨, 刘智微, 钟小仙, 吴娟子, 张建丽, 潘玉梅. 海滨雀稗自交结实突变体及野生型幼穗组织的转录组分析[J]. 草业学报, 2019, 28(5): 132-142. |
| [4] | 刘天增, 谢新春, 张巨明. 海滨雀稗60Co-γ辐射诱变突变体筛选[J]. 草业学报, 2017, 26(7): 62-70. |
| [5] | 周香艳, 张宁, 刘柏林, 裴瑞芳, 司怀军, 王蒂. ARF基因干扰表达对不同发育阶段和贮藏条件马铃薯酶活性的影响[J]. 草业学报, 2016, 25(4): 133-139. |
| [6] | 于景金, 李冉, 刘梦娴, 杨志民. 暖季型与冷季型草坪草差异响应干旱及旱后复水的生理生态机制[J]. 草业学报, 2016, 25(11): 86-93. |
| [7] | 裴瑞芳, 刘英, 刘柏林, 张宁, 司怀军, 王蒂. 马铃薯ARF基因RNAi载体的遗传转化及对其试管薯生理特性的影响[J]. 草业学报, 2015, 24(2): 142-147. |
| [8] | 贾新平,邓衍明,孙晓波,梁丽建. 盐胁迫对海滨雀稗生长和生理特性的影响[J]. 草业学报, 2015, 24(12): 204-212. |
| [9] | 贾新平,叶晓青,梁丽建,邓衍明,孙晓波,佘建明. 基于高通量测序的海滨雀稗转录组学研究[J]. 草业学报, 2014, 23(6): 242-252. |
| [10] | 宋辉,南志标,蔡小宁,钟小仙,顾洪如. 海滨雀稗液泡膜H+-PPase(PvVP1)5'端的克隆和序列分析[J]. 草业学报, 2014, 23(5): 168-174. |
| [11] | 钟小仙,刘智微,常盼盼,吴娟子,张建丽. 秋水仙素诱导获得自交结实的海滨雀稗体细胞突变体[J]. 草业学报, 2013, 22(6): 205-212. |
| [12] | 常盼盼,钟小仙,刘智微. 海滨雀稗体细胞突变体SP2008-3的特异性分析[J]. 草业学报, 2012, 21(6): 207-212. |
| [13] | 方永丰,李永生,彭云玲,王芳,王威,穆延召,王汉宁. 农杆菌介导Chi-linker-Glu融合基因和bar基因转化玉米茎尖的研究[J]. 草业学报, 2012, 21(5): 69-76. |
| [14] | 张芳,王舟,宗俊勤,刘建秀,佘建明. 农杆菌介导的假俭草遗传转化体系的建立[J]. 草业学报, 2011, 20(2): 184-192. |
| [15] | 冯波,段娇娇,伍国强,王锁民. 多浆旱生植物霸王高频组培再生体系的建立[J]. 草业学报, 2010, 19(6): 140-146. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||