

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2025, Vol. 34 ›› Issue (2): 174-183.DOI: 10.11686/cyxb2024147
• 研究论文 • 上一篇
收稿日期:2024-04-29
修回日期:2024-06-17
出版日期:2025-02-20
发布日期:2024-11-27
通讯作者:
王森山
作者简介:E-mail: wangsenshan@gsau.edu.cn基金资助:
Rui MA( ), Yi-ting WU, Yan LI, Zhan-zi YU, Lei LIU, Sen-shan WANG(
), Yi-ting WU, Yan LI, Zhan-zi YU, Lei LIU, Sen-shan WANG( )
)
Received:2024-04-29
Revised:2024-06-17
Online:2025-02-20
Published:2024-11-27
Contact:
Sen-shan WANG
摘要:
为探究豌豆蚜ApCtsk1基因的生理功能,评估其作为豌豆蚜防治靶标的潜力,从而为防控策略的制定提供理论依据。本研究以豌豆蚜为对象,利用NCBI、ExPASy Proteomics Server、MEGA 11等在线网站和软件分析ApCtsk1的氨基酸序列特征并构建系统发育树;采用RT-qPCR技术解析其在不同发育阶段、不同组织的表达模式;通过RNAi研究ApCtsk1在豌豆蚜个体发育和繁殖中的生物学功能。结果表明:豌豆蚜ApCtsk1与半翅目蚜科昆虫的半胱氨酸蛋白酶聚为一支,其在4龄若蚜期显著高表达,并在成虫腹部、卵巢、表皮表达量较高。RNAi干扰3龄若蚜的ApCtsk1后,与对照组相比,豌豆蚜死亡率升高至22.22%,体长体宽分别减小12.56%、13.22%,体色异常,行动缓慢。此外,经dsApCtsk1处理的蚜虫出现蜕皮失败的致死现象,且干扰后豌豆蚜繁殖力也受到影响,表现为产蚜时间的推迟和一段时间产蚜量的降低。由此可见,ApCtsk1在豌豆蚜个体发育、存活和繁殖过程中具有重要的作用。
马瑞, 吴宜亭, 李岩, 于展姿, 刘磊, 王森山. 豌豆蚜组织蛋白酶K基因(ApCtsk1)的鉴定及功能研究[J]. 草业学报, 2025, 34(2): 174-183.
Rui MA, Yi-ting WU, Yan LI, Zhan-zi YU, Lei LIU, Sen-shan WANG. Identification and functional analysis of ApCtsk1 encoding cathepsin K in Acyrthosiphon pisum[J]. Acta Prataculturae Sinica, 2025, 34(2): 174-183.
基因 名称 Gene name | 引物序列 Primer sequence (5′-3′) | 产物 大小 Product size (bp) | 扩增效率 Amplification efficiency (%) |
|---|---|---|---|
| EF1α | CTGTGCTTATTGTCGCTGCT TCGCTGTATGGTGGTTCAGT | 157 | 108.25 |
| RPL7 | TTGAAGAGCGTAAGGGAACTG TATTGGTGATTGGAATGCGTTG | 76 | 97.89 |
| ApCtsk1 | ACTGGTACCATCGAAGGAGC GCCATGCTTCTCAATCCAAGA | 153 | 101.71 |
表1 豌豆蚜ApCtsk1基因qPCR的引物序列
Table 1 Primer sequence of qPCR for ApCtsk1 gene of A. pisum
基因 名称 Gene name | 引物序列 Primer sequence (5′-3′) | 产物 大小 Product size (bp) | 扩增效率 Amplification efficiency (%) |
|---|---|---|---|
| EF1α | CTGTGCTTATTGTCGCTGCT TCGCTGTATGGTGGTTCAGT | 157 | 108.25 |
| RPL7 | TTGAAGAGCGTAAGGGAACTG TATTGGTGATTGGAATGCGTTG | 76 | 97.89 |
| ApCtsk1 | ACTGGTACCATCGAAGGAGC GCCATGCTTCTCAATCCAAGA | 153 | 101.71 |
基因 Gene | 引物方向 Primer direction (5′-3′) | 核苷酸序列 Nucleotide sequence (5′-3′) | 产物长度 Product size (bp) |
|---|---|---|---|
| dsGFP | 上游Forward | TAATACGACTCACTATAGGGCAGTTCTTGTTGAATTAGATG | 436 |
| 下游Reverse | TAATACGACTCACTATAGGGTTTGGTTTGTCTCCCATGATG | ||
| dsApCtsk1 | 上游Forward | TAATACGACTCACTATAGGGTGAATCTTTCCCTGGACCTGG | 541 |
| 下游Reverse | TAATACGACTCACTATAGGGATTGCCAAAACCCCAACTGC |
表2 dsRNA引物
Table 2 dsRNA primers
基因 Gene | 引物方向 Primer direction (5′-3′) | 核苷酸序列 Nucleotide sequence (5′-3′) | 产物长度 Product size (bp) |
|---|---|---|---|
| dsGFP | 上游Forward | TAATACGACTCACTATAGGGCAGTTCTTGTTGAATTAGATG | 436 |
| 下游Reverse | TAATACGACTCACTATAGGGTTTGGTTTGTCTCCCATGATG | ||
| dsApCtsk1 | 上游Forward | TAATACGACTCACTATAGGGTGAATCTTTCCCTGGACCTGG | 541 |
| 下游Reverse | TAATACGACTCACTATAGGGATTGCCAAAACCCCAACTGC |
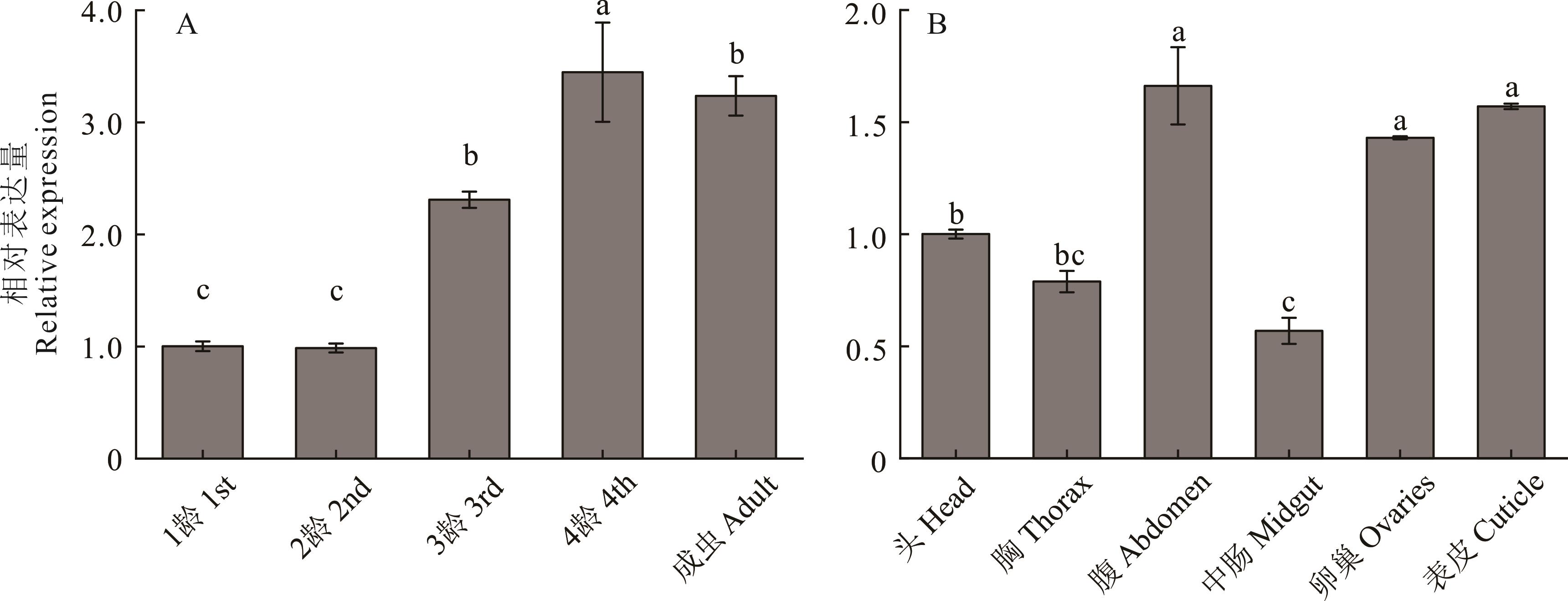
图3 豌豆蚜不同发育阶段、不同组织的ApCtsk1表达量A:ApCtsk1在豌豆蚜不同龄期的表达量;B:ApCtsk1在豌豆蚜成蚜不同组织的表达量;不同字母表示差异显著(P<0.05)。A: The expression level of ApCtsk1 in different instars of A. pisum; B: The expression level of ApCtsk1 in different tissues of adult A. pisum; Different letters indicate significant differences at the 0.05 level.
Fig.3 The expression of ApCtsk1 in different developmental stages and different tissues of A. pisum
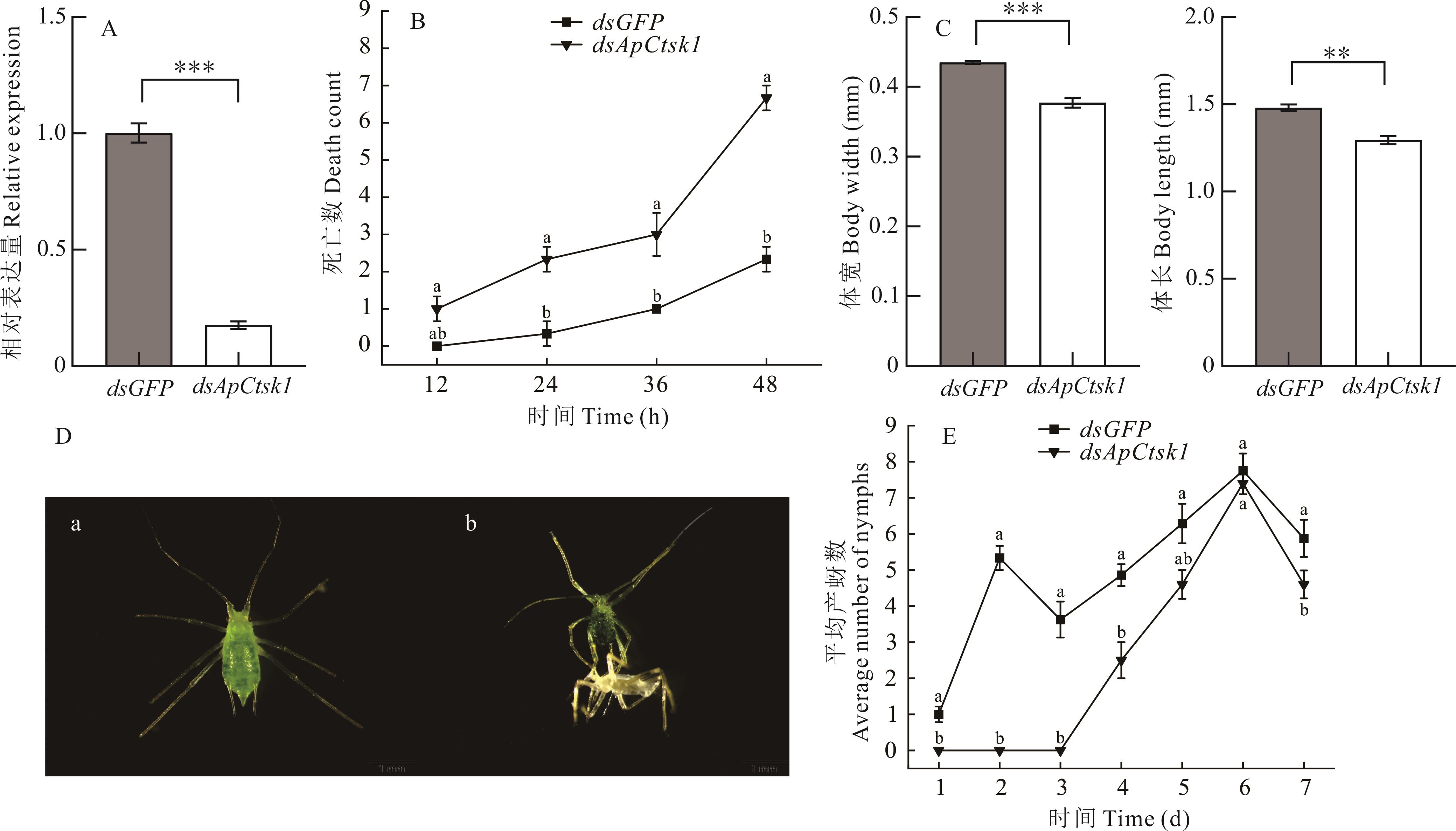
图4 RNAi ApCtsk1处理后对豌豆蚜的影响A:豌豆蚜ApCtsk1的沉默效率;B:RNAi ApCtsk1对豌豆蚜死亡数的影响;C:RNAi ApCtsk1对豌豆蚜体长、体宽的影响;D:RNAi ApCtsk1处理后豌豆蚜出现的致死表型,a经dsGFP处理,b经dsApCtsk1处理;E:RNAi ApCtsk1对豌豆蚜单日平均产蚜力的影响。相对表达量和体长体宽采用独立样本T检验进行差异显著性分析,*表示显著差异(P<0.05),**表示极显著差异(P<0.01),***表示极其显著差异(P<0.001)。死亡数和平均产蚜数采用单因素方差分析,不同字母表示差异显著(P<0.05)。A: The silencing efficiency of ApCtsk1; B: The effect of RNAi ApCtsk1 on the death count of A. pisum; C: The effect of RNAi ApCtsk1 on the body length and body width of A. pisum; D: The lethal phenotype of A. pisum after RNAi ApCtsk1, a, treated with dsGFP, b, treated with dsApCtsk1; E: Effects of RNAi ApCtsk1 on the average daily fecundity of A. pisum. The relative expression and body length and body width were analyzed by independent sample T-test, * represents significant difference (P<0.05), ** represents extremely significant difference (P<0.01), *** represents extremely significant difference (P<0.001). The number of deaths and the average number of aphids were analyzed by one-way analysis of variance, and different letters indicated significant differences (P<0.05).
Fig.4 The effect of RNAi ApCtsk1 on A. pisum
| 1 | Mort J S, Buttle D J. Cathepsin B. The International Journal of Biochemistry & Cell Biology, 1997, 29(5): 715-720. |
| 2 | Barros N M T, Puzer L, Tersariol I L, et al. Plasma prekallikrein/kallikrein processing by lysosomal cysteine proteases. Biological Chemistry, 2004, 385(11): 1087-1091. |
| 3 | Lang T, Willinger U, Holzer G, et al. Soluble cathepsin-L: A marker of bone resorption and bone density. Journal of Laboratory and Clinical Medicine, 2004, 144(3): 163-166. |
| 4 | Lu S Y, Ren H L, Liu Z S, et al. The progress of study on the cathepsin B. Journal of Hebei Normal University (Natural Science Edition), 2004, 28(3): 306-309. |
| 卢士英, 任洪林, 柳增善, 等. 组织蛋白酶B研究进展. 河北师范大学学报(自然科学版), 2004, 28(3): 306-309. | |
| 5 | Chen L, Zhang M, Sun L, et al. Identification and expressional analysis of two cathepsins from half-smooth tongue sole (Cynoglossus semilaevis). Fish & Shellfish Immunology, 2011, 31(6): 1270-1277. |
| 6 | Pan G Z. Identification and functional analysis of Cathepsin L in silkworm (Bombyx mori). Chongqing: Southwest University, 2018. |
| 潘光照. 家蚕Cathepsin L的鉴定及功能初探. 重庆: 西南大学, 2018. | |
| 7 | Dalton J P, Neill S O, Stack C, et al. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. International Journal for Parasitology, 2003, 33(11): 1173-1181. |
| 8 | Liu J, Shi G P, Zhang W Q, et al. Cathepsin L function in insect moulting: molecular cloning and functional analysis in cotton bollworm, Helicoverpa armigera.Insect Molecular Biology, 2006, 15(6): 823-834. |
| 9 | Wang L F, Chai L Q, He H J, et al. A cathepsin L-like proteinase is involved in moulting and metamorphosis in Helicoverpa armigera. Insect Molecular Biology, 2010, 19(1): 99-111. |
| 10 | Turk V, Stoka V, Vasiljeva O, et al. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2012, 1824(1): 68-88. |
| 11 | Patil A D, Freyer A J, Carte B, et al. Haploscleridamine, a novel tryptamine-derived alkaloid from a sponge of the order Haplosclerida: an inhibitor of cathepsin K. Journal of Natural Products, 2002, 65(4): 628-629. |
| 12 | Lecaille F, Kaleta J, Brömme D, et al. Human and parasitic papain-like cysteine proteases: their role in physiology and pathology and recent developments in inhibitor design. Chemical Reviews, 2002, 102(12): 4459-4488. |
| 13 | He W T, Liu K, Sun J X, et al. Research advance of cathepsin K and the treatment of osteoporosis. Chinese Journal of Osteoporosis, 2008, 14(9): 670-673. |
| 何伟涛, 刘康, 孙金谞, 等. 组织蛋白酶K与骨质疏松症治疗的研究进展. 中国骨质疏松杂志, 2008, 14(9): 670-673. | |
| 14 | Costa A G, Cusano N E, Silva B C, et al. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nature Reviews Rheumatology, 2011, 7(8): 447-456. |
| 15 | Novinec M, Lenarčič B. Cathepsin K: a unique collagenolytic cysteine peptidase. Biological Chemistry, 2013, 394(9): 1163-1179. |
| 16 | Tesfaye A, Wale M, Azerefegne F, et al. Acyrthosiphon pisum(Harris) (Homoptera: Aphididae) feeding preference and performance on cool-season food legumes in northwestern Ethiopia. International Journal of Pest Management, 2013, 59(4): 319-328. |
| 17 | Soleimani S, Madadi H. Seasonal dynamics of: the pea aphid, Acyrthosiphon pisum (Harris), its natural enemies the seven spotted lady beetle Coccinella septempunctata Linnaeus and variegated lady beetle Hippodamia variegata Goeze, and their parasitoid Dinocampus coccinellae (Schrank). Journal of Plant Protection Research, 2015, 55(4): 421-428. |
| 18 | Aznar Fernández T, Cimmino A, Masi M, et al. Antifeedant activity of long-chain alcohols, and fungal and plant metabolites against pea aphid (Acyrthosiphon pisum) as potential biocontrol strategy.Natural Product Research, 2018, 33(17): 2471-2479. |
| 19 | Srinivasan D G, Abdelhady A, Stern D L, et al. Gene expression analysis of parthenogenetic embryonic development of the pea aphid, Acyrthosiphon pisum, suggests that aphid parthenogenesis evolved from meiotic oogenesis. PLoS One, 2014, 9(12): e115099. |
| 20 | Paudel S, Bechinski E J, Stokes B S, et al. Deriving economic models for pea aphid (Hemiptera: Aphididae) as a direct-pest and a virus-vector on commercial lentils. Journal of Economic Entomology, 2018, 111(5): 2225-2232. |
| 21 | Rashed A, Feng X, Sean M, et al. Vector-borne viruses of pulse crops, with a particular emphasis on north American cropping system. Annals of the Entomological Society of America, 2018, 111(4): 205-227. |
| 22 | Wang D, Deng J, Pei Y F, et al. Identification and virulence characterization of entomopathogenic fungus Lecanicillium attenuatum against the pea aphid Acyrthosiphon pisum (Hemiptera: Aphididae). Applied Entomology and Zoology, 2017, 52(3): 511-518. |
| 23 | Lu W N, Kuo M H. Life table and heat tolerance of Acyrthosiphon pisum (Hemiptera: Aphididae) in subtropical Taiwan. Entomological Science, 2008, 11(3): 273-279. |
| 24 | Bhatnagar A. Efficacy and economics of some insecticides and a neem formulation on incidence of pea aphid (Acrythosiphum pisum) on pea, Pisum sativum. Annals of Plant Protection Sciences, 1996, 4(2): 131-133. |
| 25 | Saikhedkar N, Summanwar A,JoshiR, et al. Cathepsins of Lepidopteran insects: Aspects and prospects. Insect Biochemistry and Molecular Biology, 2015, 64(2015): 51-59. |
| 26 | Cheng X A, Lin X W, Jiang X H, et al. cDNA cloning, prokaryotic expression and polyclonal antibody preparation of cathepsin L in Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Entomologica Sinica, 2018, 61(4): 410-422. |
| 程杏安, 林贤伟, 蒋旭红, 等. 草地贪夜蛾组织蛋白酶L的基因克隆、原核表达及多克隆抗体制备. 昆虫学报, 2018, 61(4): 410-422. | |
| 27 | Yang K M, Bae E, Ahn S G, et al. Co-chaperone BAG2 determines the pro-oncogenic role of cathepsin B in triple-negative breast cancer cells. Cell Reports, 2017, 21(10): 2952-2964. |
| 28 | Pym A, Singh K S, Nordgren Å, et al. Host plant adaptation in the polyphagous whitefly, Trialeurodes vaporariorum, is associated with transcriptional plasticity and altered sensitivity to insecticides. BMC Genomics, 2019, 20(1): 996. |
| 29 | Pyati P, Bandani A R, Fitches E, et al. Protein digestion in cereal aphids (Sitobion avenae) as a target for plant defence by endogenous proteinase inhibitors. Journal of Insect Physiology, 2011, 57(7): 881-891. |
| 30 | Cristofoletti P T, Ribeiro A F, Deraison C, et al. Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acyrthosiphon pisum. Journal of Insect Physiology, 2003, 49(1): 11-24. |
| 31 | Ramsey J S, Wilson A C C, de Vos M, et al. Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics, 2007, 8(1): 423-450. |
| 32 | Deraison C, Darboux I, Duportets L, et al. Cloning and characterization of a gut-specific cathepsin L from the aphid Aphis gossypii. Insect Molecular Biology, 2004, 13(2): 165-177. |
| 33 | Sapountzis P, Duport G, Balmand S, et al. New insight into the RNA interference response against cathepsin-L gene in the pea aphid, Acyrthosiphon pisum: Molting or gut phenotypes specifically induced by injection or feeding treatments.Insect Biochemistry and Molecular Biology, 2014, 51(2014): 20-32. |
| 34 | Mathers T C, Chen Y, Kaithakottil G, et al. Rapid transcriptional plasticity of duplicated gene clusters enables a clonally reproducing aphid to colonise diverse plant species. Genome Biology, 2017, 18(1): 27-47. |
| 35 | Waterhouse A, Bertoni M. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Research, 2018, 46(W1): W296-W303. |
| 36 | Nikmanesh A, Esmailizadeh A. Comparison of genetic diversity and phylogenetic structure of BRCA1 gene of some domestic and wild sheep breeds in different countries. Animal Biotechnology, 2023, 34(9): 4746-4759. |
| 37 | Rio D C, Ares M. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protocols, 2010, 2010(6): 5439-5442. |
| 38 | Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods, 2001, 25(4): 402-408. |
| 39 | Liu J. Physiology function analysis of lysosomal cysteine proteases. Hefei: University of Science and Technology of China, 2006. |
| 刘健. 溶酶体半胱氨酸蛋白酶生理功能的研究. 合肥: 中国科学技术大学, 2006. | |
| 40 | Cai X Y, Yu J, Yu H Y, et al. Core promoter regulates the expression of cathepsin B gene in the fat body of Bombyx mori. Gene, 2014, 542(2): 232-239. |
| 41 | Guo S Y, Wu W M, Li S Y, et al. 20-hydroxyecdysone-upregulated proteases involved in Bombyx larval fat body destruction.Insect Molecular Biology, 2018, 27(6): 724-738. |
| 42 | Liao J Y. The mechanism of cathepsin affects the transmission of tomato chlorosis virus by Q Bemisia tabaci. Changsha: Hunan University, 2020. |
| 廖锦钰. 组织蛋白酶影响Q烟粉虱传播番茄褪绿病毒的机制. 长沙: 湖南大学, 2020. | |
| 43 | Liu W J, Zhang X L, Gao X K, et al. Clone and expression analysis of five cathepsin B genes in different host-specific types of Aphis gossypii Glover. Journal of Environmental Entomology, 2022, 44(4): 946-955. |
| 刘伟娇, 张肖丽, 高雪珂, 等. 5个棉蚜组织蛋白酶B基因克隆及在不同寄主专化型中的表达分析. 环境昆虫学报, 2022, 44(4): 946-955. | |
| 44 | Martynov A G, Elpidina E N, Perkin L, et al. Functional analysis of C1 family cysteine peptidases in the larval gut of Тenebrio molitor and Tribolium castaneum. BMC Genomics, 2015, 16(1): 75. |
| 45 | Zhao X, Wang J, Yagi N, et al. Occurrence of a cathepsin B-like acid cysteine proteinase in the eggs of silkworm moth, Antheraea pernyi. Comparative Biochemistry and Physiology B-Biochemistry and Molecular Biology, 1996, 113(1): 95-103. |
| 46 | Kang T, Jin R, Zhang Y, et al. Functional characterization of the aspartic proteinase cathepsin D in the beet armyworm (Spodoptera exigua). Gene, 2017, 617: 1-7. |
| 47 | Liu J P. The response of Aphis gossypii and Acyrthosiphon gossypii from Xinjiang cotton-growing region to heat stress and its molecular mechanism. Beijing: Chinese Academy of Agricultural Sciences, 2021. |
| 刘金萍. 新疆棉区棉蚜和棉长管蚜对高温胁迫的响应及分子机制. 北京: 中国农业科学院, 2021. | |
| 48 | Wei L T. Expression characteristics and RNAi effect analysis of four cysteine protease genes TccatB25, TccatL11, TccatL13 and TcPigk in Tribolium castaneum. Nanjing: Nanjing Normal University, 2019. |
| 魏璐婷. 赤拟谷盗四个半胱氨酸蛋白酶基因TccatB25、TccatL11、TccatL13和TcPigk的表达特性与RNAi效应分析. 南京: 南京师范大学, 2019. | |
| 49 | Rauf I, Asif M, Amin I, et al. Silencing cathepsin L expression reduces Myzus persicae protein content and the nutritional value as prey for Coccinella septempunctata. Insect Molecular Biology, 2019, 28(6): 785-797. |
| 50 | Carnevali O, Cionna C, Tosti L, et al. Role of cathepsins in ovarian follicle growth and maturation. General and Comparative Endocrinology, 2006, 146(3): 195-203. |
| [1] | 刘莉莉, 王月霖, 李海燕, 丰吉, 初丽爽, 杨允菲, 兰理实, 郭健. 东北退化草原恢复演替系列羊草和寸草无性系种群构件营养繁殖特征比较[J]. 草业学报, 2024, 33(7): 15-24. |
| [2] | 赵沛迪, 杨可, 蒋玉奇, 黄轲盼, 马明宇, 李开栋, 许辉, 李万宏. 日粮能量水平对绵羊睾丸发育和相关基因表达的影响[J]. 草业学报, 2024, 33(6): 219-226. |
| [3] | 丁仁翔, 刘浩, 朱科燃, 张雨, 王鑫, 杨蒙, 朱浩铖, 刘光立. 夹金山3种同域分布绿绒蒿的传粉生态学研究[J]. 草业学报, 2024, 33(1): 207-216. |
| [4] | 丰吉, 刘志扩, 李海燕, 杨允菲, 郭健. 围栏封育和长期刈割对松嫩草地羊草和野古草种群营养繁殖特征的影响[J]. 草业学报, 2023, 32(5): 50-60. |
| [5] | 张永超, 魏小星, 梁国玲, 秦燕, 刘文辉, 贾志锋, 刘勇, 马祥. 老芒麦衰老过程形态特征变化规律及对养分添加的响应[J]. 草业学报, 2022, 31(6): 101-111. |
| [6] | 骆望龙, 夏建强, 李佳欣, 孙淑范, 汪睿, 张勃. 高寒退化草地狼毒繁殖性状的选择及其适应性[J]. 草业学报, 2021, 30(4): 121-129. |
| [7] | 马亚玲, 刘辉, 刘阳, 李春杰. 两种色型豌豆蚜生物学特征对不同大豆品种的响应[J]. 草业学报, 2020, 29(3): 96-102. |
| [8] | 白乌云, 侯向阳, 武自念, 田春育, 丁勇. 羊草不同地理种群表型变异及其对根茎克隆繁殖的影响[J]. 草业学报, 2020, 29(12): 86-94. |
| [9] | 李应德, 丁婷婷, 段廷玉. AM真菌对紫花苜蓿应答蚜虫胁迫的影响[J]. 草业学报, 2020, 29(1): 155-162. |
| [10] | 宋月媛, 杨允菲. 松嫩平原林缘草地羽茅无性系构件结构与生长分析[J]. 草业学报, 2019, 28(7): 168-174. |
| [11] | 徐晓霞, 刘金平, 游明鸿, 张小晶, 谢瑞娟. 遮阴和干旱对荩草克隆生长和有性繁殖及权衡关系的影响[J]. 草业学报, 2019, 28(2): 121-132. |
| [12] | 陈林,李月飞,苏莹,宋乃平,杨新国,王磊,卞莹莹,杨丽娜. 荒漠草原不同土壤生境猪毛蒿个体大小依赖的繁殖分配[J]. 草业学报, 2018, 27(12): 79-93. |
| [13] | 王沛, 崔彦农, 高丽, 王锁民. 盐生植物小花碱茅CYP86A基因的RNAi载体构建[J]. 草业学报, 2017, 26(6): 105-110. |
| [14] | 李润红, 刘长仲. 大气CO2浓度升高对绿色型豌豆蚜生长发育和繁殖的影响[J]. 草业学报, 2017, 26(3): 111-120. |
| [15] | 郑普阳, 彭真, 王勇, 徐云虎, 贺兵, 王玉梅, 赵景瑞. 贝奥不育剂和溴敌隆抗凝血杀鼠剂对布氏田鼠种群控制作用的试验研究[J]. 草业学报, 2017, 26(12): 186-193. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||