

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2023, Vol. 32 ›› Issue (7): 49-60.DOI: 10.11686/cyxb2022425
王少鹏1( ), 刘佳1(
), 刘佳1( ), 洪军2, 林积圳2, 张义2, 史昆1, 王赞1(
), 洪军2, 林积圳2, 张义2, 史昆1, 王赞1( )
)
收稿日期:2022-10-27
修回日期:2022-11-22
出版日期:2023-07-20
发布日期:2023-05-26
通讯作者:
王赞
作者简介:E-mail: zanwang@cau.edu.cn基金资助:
Shao-peng WANG1( ), Jia LIU1(
), Jia LIU1( ), Jun HONG2, Ji-zhen LIN2, Yi ZHANG2, Kun SHI1, Zan WANG1(
), Jun HONG2, Ji-zhen LIN2, Yi ZHANG2, Kun SHI1, Zan WANG1( )
)
Received:2022-10-27
Revised:2022-11-22
Online:2023-07-20
Published:2023-05-26
Contact:
Zan WANG
摘要:
干旱是影响植物生长发育及产量的重要环境因素,PPR(pentatricopeptide repeats)家族蛋白在植物生长发育以及胁迫响应等生理过程中都具有重要作用。本研究在中苜1号紫花苜蓿中克隆到MsPPR1,利用病毒介导的基因沉默技术和烟草异源表达,在中苜1号紫花苜蓿和烟草中验证了MsPPR1在抗旱性中的功能。结果显示,MsPPR1开放阅读框包含3213 bp,编码1070个氨基酸,相对分子量为121.65 kDa,具有多个PPR重复结构域,定位于细胞质,是典型的PPR蛋白家族成员。MsPPR1主要表达在叶中,其次是茎和根,花中最少;其表达量受自然干旱、甘露醇和脱落酸处理的诱导。通过病毒诱导基因沉默技术在紫花苜蓿中降低了MsPPR1的表达,干旱胁迫下,沉默植株更加萎蔫,相对含水量显著降低,相对电解质渗透率显著升高,显著降低了紫花苜蓿的抗旱性。在烟草中异源超表达MsPPR1,显著增强了转基因烟草的抗旱性,干旱胁迫下,丙二醛含量显著降低,脯氨酸含量增加。以上结果均表明MsPPR1是紫花苜蓿抗旱性的正调控因子,本研究为紫花苜蓿抗旱分子育种提供了候选基因。
王少鹏, 刘佳, 洪军, 林积圳, 张义, 史昆, 王赞. 紫花苜蓿MsPPR1基因的克隆及抗旱功能分析[J]. 草业学报, 2023, 32(7): 49-60.
Shao-peng WANG, Jia LIU, Jun HONG, Ji-zhen LIN, Yi ZHANG, Kun SHI, Zan WANG. Cloning and function analysis of MsPPR1 in alfalfa under drought stress[J]. Acta Prataculturae Sinica, 2023, 32(7): 49-60.
| 引物名称Primers | 引物序列Primer sequence (5'-3') |
|---|---|
| MsPPR1-F | ATGTTGTTCTCCTCAACAAAACCC |
| MsPPR1-R | TCAGTTCATAGACTCTGTCATGTCTGA |
| MsPPR1-GFP-F | TTAATTAAATGTTGTTCTCCTCAACAAAA |
| MsPPR1-GFP-R | GGCGCGCCGTTCATAGACTCTGTC |
| pBI121-MsPPR1-F | GAGAACACGGGGGACTCTAGAATGTTGTTCTCCTCAAC |
| pBI121-MsPPR1-R | AAGGGACTGACCACCCGGGGAGTTCATAGACTCTGT |
| pTRV2-MsPPR1-F | TGAGTAAGGTTACCGAATTCTGGGTTTGTGGGTATGTTGA |
| pTRV2-MsPPR1-R | GTGAGCTCGGTACCGGATCCTGAGAACTCATCCGCCTCCT |
| MsPPR1-qPCR-F | GTTTCTAGTTCCGTGGTTTCGGCGT |
| MsPPR1-qPCR-R | AAACTTCATCAACCCTCCCCAACTT |
| MsActin-qPCR-F | CAAAAGATGGCAGATGCTGAGGAT |
| MsActin-qPCR-R | CATGACACCAGTATGACGAGGTCG |
表 1 试验所用引物
Table 1 Primers used in the study
| 引物名称Primers | 引物序列Primer sequence (5'-3') |
|---|---|
| MsPPR1-F | ATGTTGTTCTCCTCAACAAAACCC |
| MsPPR1-R | TCAGTTCATAGACTCTGTCATGTCTGA |
| MsPPR1-GFP-F | TTAATTAAATGTTGTTCTCCTCAACAAAA |
| MsPPR1-GFP-R | GGCGCGCCGTTCATAGACTCTGTC |
| pBI121-MsPPR1-F | GAGAACACGGGGGACTCTAGAATGTTGTTCTCCTCAAC |
| pBI121-MsPPR1-R | AAGGGACTGACCACCCGGGGAGTTCATAGACTCTGT |
| pTRV2-MsPPR1-F | TGAGTAAGGTTACCGAATTCTGGGTTTGTGGGTATGTTGA |
| pTRV2-MsPPR1-R | GTGAGCTCGGTACCGGATCCTGAGAACTCATCCGCCTCCT |
| MsPPR1-qPCR-F | GTTTCTAGTTCCGTGGTTTCGGCGT |
| MsPPR1-qPCR-R | AAACTTCATCAACCCTCCCCAACTT |
| MsActin-qPCR-F | CAAAAGATGGCAGATGCTGAGGAT |
| MsActin-qPCR-R | CATGACACCAGTATGACGAGGTCG |
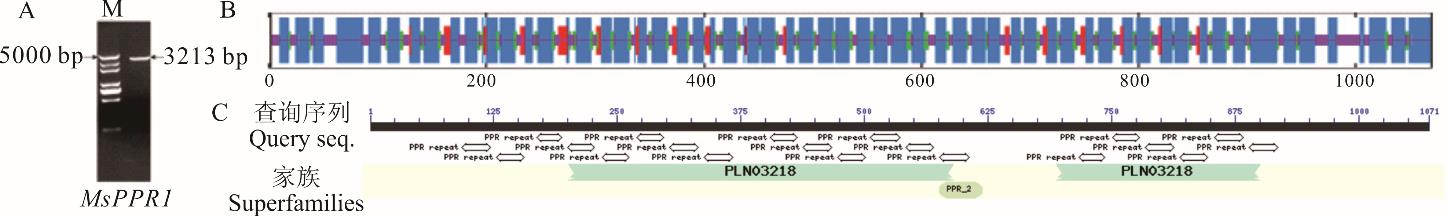
图1 MsPPR1的生物信息学分析A: MsPPR1的克隆Clone of MsPPR1, M: DNA分子量标准DNA marker; B: MsPPR1蛋白质二级结构的预测Prediction of the secondary structure of MsPPR1; C: MsPPR1的保守结构域The conserved structural domains of MsPPR1.
Fig.1 Bioinformatic analysis of the deduced MsPPR1
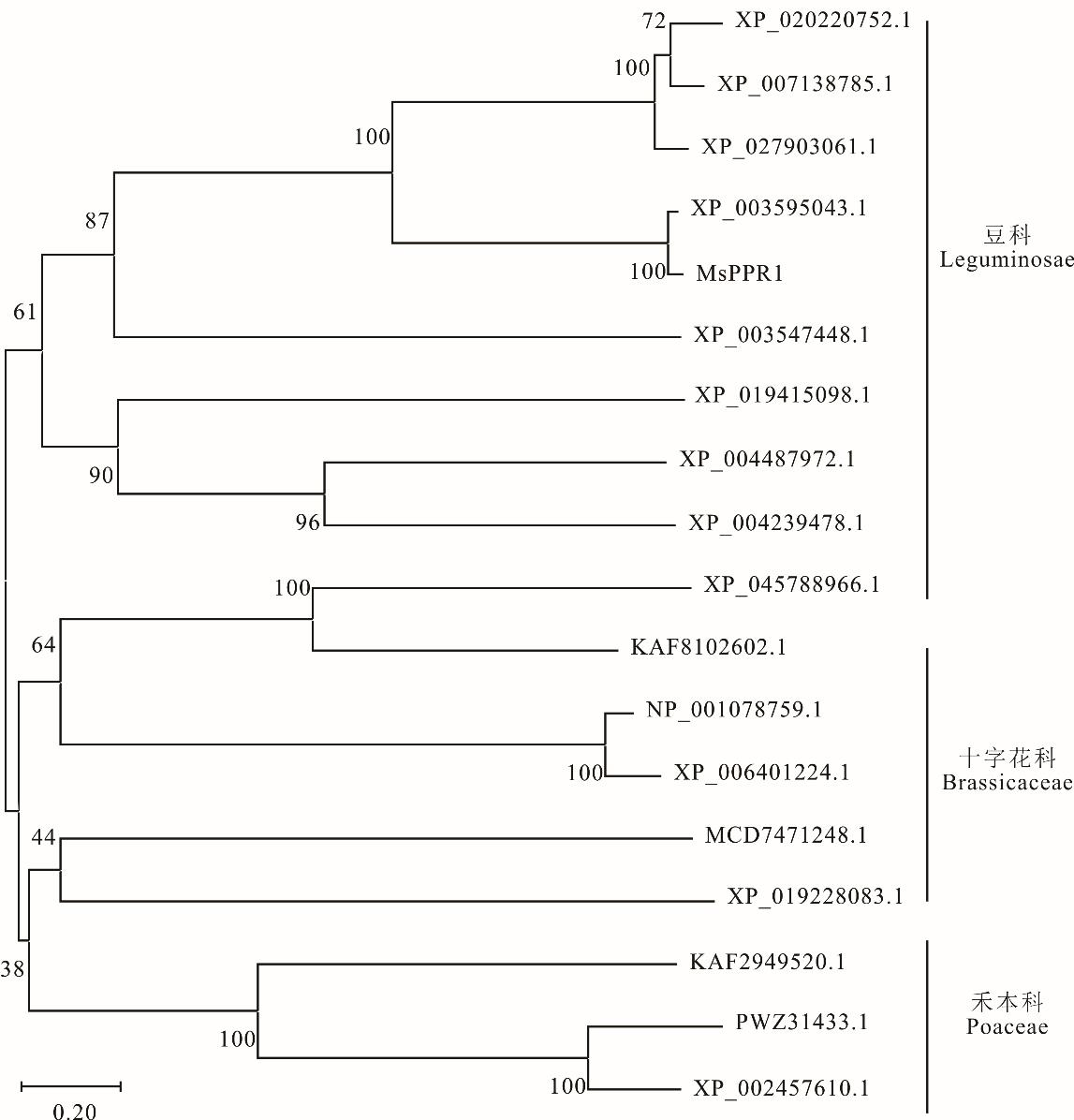
图2 多物种中PPR家族蛋白的系统进化树分析图为MsPPR1与其他植物PPR蛋白进化树分析。The figure shows the phylogenetic tree analysis of MsPPR1 and other plant PPR proteins. XP_020220752.1: 木豆C. cajan; XP_007138785.1: 菜豆P. vulgaris; XP_027903061.1: 豇豆V. unguiculata; XP_003595043.1: 蒺藜苜蓿M. truncatula; XP_003547448.1: 大豆G. max; XP_019415098.1: 狭叶羽扇豆L. angustifolius; XP_004487972.1: 鹰嘴豆C. arietinum; XP_004239478.1: 番茄S. lycopersicum; XP_045788966.1: 红三叶T. pratense; KAF8102602.1: 白芥S. alba; NP_001078759.1: 拟南芥A. thaliana; XP_006401224.1: 盐芥E. salsugineum; MCD7471248.1: 曼陀罗D. stramonium; XP_019228083.1: 烟草N. benthamiana; KAF2949520.1: 粳稻O. sativa Japonica Group; PWZ31433.1: 玉米Z. mays; XP_002457610.1: 高粱S. bicolor.
Fig.2 Phylogenetic tree analysis of PPR family proteins in multiple species

图4 紫花苜蓿MsPPR1相对表达量分析A: 不同组织中的表达The relative expression of MsPPR1 in the indicated tissues of alfalfa; B: 自然干旱处理下的表达The relative expression of MsPPR1 under nature drought; C: 甘露醇处理下的表达The relative expression of MsPPR1 under mannitol (200 mmol·L-1); D: 脱落酸处理下的表达The relative expression of MsPPR1 under ABA (100 μmol·L-1).不同小写字母表示处理之间差异显著(P<0.05)。Different lowercase letters indicate significant differences among treatments (P<0.05). 下同The same below.
Fig.4 Analysis of relative expression of MsPPR1 in alfalfa
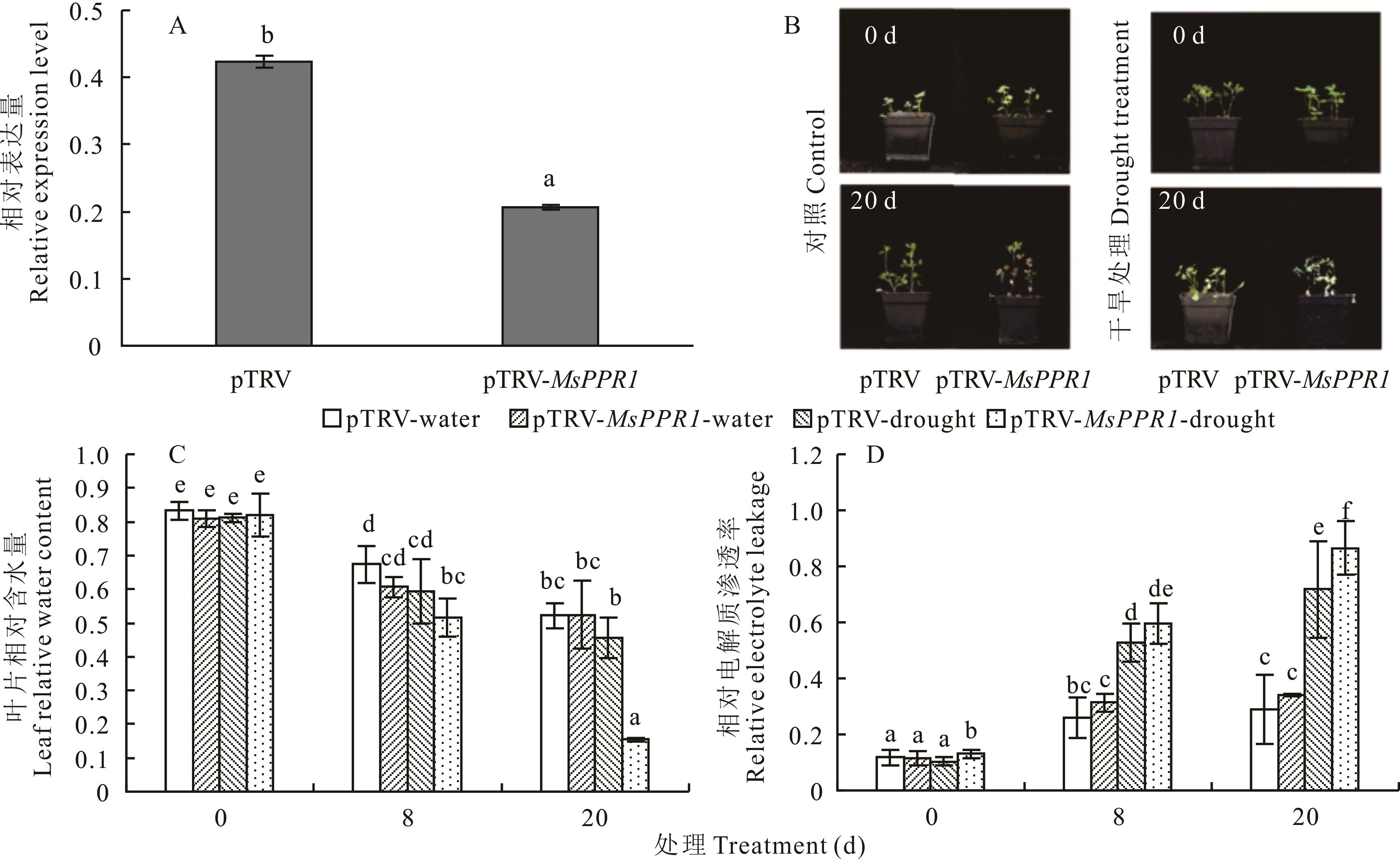
图5 病毒诱导沉默下MsPPR1表达量及紫花苜蓿干旱表型A: MsPPR1在沉默植株及对照植株中的表达量The relative expression of MsPPR1 in silent plants and control plants; B: 对照和干旱情况下0和20 d植株情况Plants in 0 and 20 days under control and drought conditions; C: 干旱处理情况下0、8、20 d植株叶片相对含水量Leaf relative leaf water content of plants in 0, 8 and 20 days under drought treatment。pTRV-water、pTRV-drought、pTRV-MsPPR1-water和pTRV-MsPPR1-drought分别为对照植株正常浇水、对照植株干旱处理、沉默MsPPR1植株正常浇水和沉默MsPPR1植株干旱处理。pTRV-water, pTRV-drought, pTRV-MsPPR1-water, and pTRV-MsPPR1-drought represent control plants water treatment, control plants drought treatment, silent MsPPR1 plants water treatment, silent MsPPR1 plants drought treatment respectively. D: 干旱处理情况下0、8、20 d植株相对电解质渗透率Relative electrolyte leakage of plants in 0, 8 and 20 days after drought treatment.
Fig.5 Expression level of MsPPR1 and phenotype under drought stress in alfalfa by virus-induced gene silencing

图6 不同株系烟草MsPPR1相对表达量及其表型A: 转基因烟草的验证The verification of genetically modified tobacco, M:DNA分子量标准DNA marker; B: 转基因烟草中MsPPR1相对表达量The relative expression of MsPPR1 in transgenic tobacco. WT、OE-1、OE-2、OE-3、OE-4、OE-5和OE-6 分别为野生型、过表达株系1、过表达株系2 、过表达株系3、过表达株系4、过表达株系5和过表达株系6,下同。WT,OE-1,OE-2, OE-3 OE-4,OE-5 and OE-6 were wild type,overexpressed line 1,overexpressed line 2, overexpressed line 3, overexpressed line 4, overexpressed line 5 and overexpressed line 6, the same below; C: 干旱处理情况下转基因烟草的表型The phenotype of transgenic tobacco under drought treatment.
Fig.6 Relative expression and phenotype of MsPPR1 in different tobacco strains
| 1 | Wang W Z, Ren Y J, Miao Y. Roles of PPR proteins in plant growth and development. Journal of Tropical and Subtropical Botany, 2019, 27(2): 225-234. |
| 王婉珍, 任育军, 缪颖. PPR蛋白在植物生长发育中的作用. 热带亚热带植物学报, 2019, 27(2): 225-234. | |
| 2 | Jiang S C, Mei C, Wang X F, et al. Expression analysis of Arabidopsis SOAR1 in response to ABA and osmotic stress. Journal of Henan Agricultural Sciences, 2016, 45(2): 29-34, 48. |
| 姜上川, 梅超, 王小芳, 等. 拟南芥SOAR1基因响应ABA与渗透胁迫的表达分析. 河南农业科学, 2016, 45(2): 29-34, 48. | |
| 3 | Emami H, Kempken F. PRECOCIOUS1 (POCO1), a mitochondrial pentatricopeptide repeat protein affects flowering time in Arabidopsis thaliana. The Plant Journal, 2019, 100(2): 265-278. |
| 4 | Su H G, Li B, Song X Y, et al. Genome-wide analysis of the DYW subgroup PPR gene family and identification of GmPPR4 responses to drought stress. International Journal of Molecular Sciences, 2019, 20(22): 5667. |
| 5 | Luo Z, Xiong J, Xia H, et al. Pentatricopeptide repeat gene-mediated mitochondrial RNA editing impacts on rice drought tolerance. Frontiers in Plant Science, 2022, 13: 926285. |
| 6 | Wei K, Han P. Pentatricopeptide repeat proteins in maize. Molecular Breeding, 2016, 36(12): 170. |
| 7 | Liu Y, Jiang D, Yan J, et al. ABA-insensitivity of alfalfa (Medicago sativa L.) during seed germination associated with plant drought tolerance. Environmental and Experimental Botany, 2022, 203: 105069. |
| 8 | Holsters M, de Waele D, Depicker A, et al. Transfection and transformation of Agrobacterium tumefaciens. Molecular and General Genetics, 1978, 163(2): 181-187. |
| 9 | Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. The Plant Journal, 2000, 22(6): 543-551. |
| 10 | Kenneth J, Thomas D. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔ CT method. Methods, 2001, 25(4): 402-408. |
| 11 | Richard E, Gail E. Rapid estimates of relative water content. Plant Physiology, 1974, 53(2): 258-260. |
| 12 | Blum A, Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Science, 1981, 21(1): 43-47. |
| 13 | Li Y. Cloning and analysis of an inducement-responsive promoter MsZPP of Medicago sativa L. Beijing: China Academy of Agricultural Sciences, 2012. |
| 李燕. 紫花苜蓿诱导表达启动子MsZPP的克隆及功能分析. 北京: 中国农业科学院, 2012. | |
| 14 | Liu J J, Zhang D Z, Zhang Y B, et al. Dynamic and comparative transcriptome analyses reveal key factors contributing to cadmium tolerance in broomcorn millet. International Journal of Molecular Science, 2022, 23(11): 6148. |
| 15 | Li P, Kong W, Wu X, et al. Volatile organic compounds of the plant growth-promoting rhizobacteria JZ-GX1 enhanced the tolerance of Robinia pseudoacacia to salt stress. Frontiers in Plant Science, 2021, 12: 753332. |
| 16 | Liang H, Wei B, Chen J, et al. Evaluation of the drought resistance in alfalfa germplasm by chlorophyll fluorescence parameters at seedling stage. Acta Agrestia Sinica, 2020, 28(1): 45-55. |
| 梁欢, 韦宝, 陈静, 等. 基于叶绿素荧光参数的紫花苜蓿种质苗期抗旱性评价. 草地学报, 2020, 28(1): 45-55. | |
| 17 | Wang Z N, Li L H, Xu R H, et al. Advances in mitochondrial proteins responding to stresses in plants. Plant Physiology Journal, 2018, 54(2): 221-231. |
| 王忠妮, 李鲁华, 徐如宏, 等. 响应植物逆境胁迫的线粒体蛋白研究进展. 植物生理学报, 2018, 54(2): 221-231. | |
| 18 | Yuan H, Liu D. Functional disruption of the pentatricopeptide protein SLG1 affects mitochondrial RNA editing, plant development, and responses to abiotic stresses in Arabidopsis. The Plant Journal, 2012, 70(3): 432-444. |
| 19 | Liu J M, Zhao J Y, Lu P P, et al. The E-subgroup pentatricopeptide repeat protein family in Arabidopsis thaliana and confirmation of the responsiveness PPR96 to abiotic stresses. Frontiers in Plant Science, 2016, 7: 1825. |
| 20 | Meierhoff K, Felder S, Nakamura T, et al. HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. The Plant Cell, 2003, 15(6): 1480-1495. |
| 21 | Okuda K, Myouga F, Motohashi R, et al. Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proceedings of the National Academy of Sciences, 2007, 104: 8178-8183. |
| 22 | Yamazaki H, Tasaka M, Shikanai T. PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression in Arabidopsis. The Plant Journal, 2004, 38(1): 152-163. |
| 23 | Chen H, Zeng Y, Yang Y, et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nature Communications, 2020, 11(1): 2494. |
| 24 | Hao Y Y, Zhao X Q, Huang F D, et al. The role of PPR proteins in posttranscriptional regulation of organelle components in plants. Hereditas (Beijing), 2021, 43(11): 1050-1065. |
| 郝媛媛, 赵向前, 黄福灯, 等. PPR蛋白在植物细胞器组分转录后调控中的作用机制. 遗传, 2021, 43(11): 1050-1065. | |
| 25 | Fisk D G, Walker M B, Barkan A. Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. The EMBO Journal, 1999, 18(9): 2621-2630. |
| 26 | Gothandam K M, Kim E, Cho H, et al. OsPPR1, a pentatricopeptide repeat protein of rice is essential for the chloroplast biogenesis. Plant Molecular Biology, 2005, 58(3): 421-433. |
| 27 | Bentolila S, Alfonso A A, Hanson M R. A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proceedings of the National Academy of Sciences, 2002, 99(16): 10887-10892. |
| 28 | Yao C J, Guo S M, Ma Y C, et al. Effect of drought stress on characteristics of photosynthesis and chlorophyll fluorescence of four species of Cassia. Pratacultural Science, 2017, 34(9): 1880-1888. |
| 姚春娟, 郭圣茂, 马英超, 等. 干旱胁迫对4种决明属植物光合作用和叶绿素荧光特性的影响. 草业科学, 2017, 34(9): 1880-1888. | |
| 29 | Qin D D, Dong J, Xu F C, et al. Identification of barley germplasms with high photosynthesis efficiency and correlation analysis between photosynthesis and yield. Modern Agricultural Science and Technology, 2017(23): 38-40, 44. |
| 秦丹丹, 董静, 许甫超, 等. 大麦高光效种质资源的筛选及光合特性与产量的相关性分析. 现代农业科技, 2017(23): 38-40, 44. | |
| 30 | Guo Y, Wang Z P, Lv Y C, et al. Evaluation of drought resistance and excellent germplasm screening in different Triticeae crops. Journal of Triticeae Crops, 2022, 42(10): 1208-1219. |
| 郭莹, 王振平, 吕迎春, 等. 不同麦类种质抗旱性评价及优异种质筛选. 麦类作物学报, 2022, 42(10): 1208-1219. | |
| 31 | Song Q Q, Zhu P J, He J, et al. Effect of low temperature on photosynthetic physiological characteristics of different Artocarpus heterophyllus Lam. germplasm resources. Agricultural Research and Application, 2021, 34(6): 7-13. |
| 宋奇琦, 朱鹏锦, 何江, 等. 低温对不同菠萝蜜种质资源光合生理特性的影响. 农业研究与应用, 2021, 34(6): 7-13. |
| [1] | 凌文卿, 张磊, 李珏, 冯启贤, 李妍, 周燚, 刘一佳, 阳伏林, 周晶. 布氏乳杆菌和不同糖类联用对紫花苜蓿青贮营养成分、发酵品质、瘤胃降解率及有氧稳定性的影响[J]. 草业学报, 2023, 32(7): 122-134. |
| [2] | 王文娟, 师尚礼, 何龙, 武蓓, 刘旵旵. 干旱胁迫下多胺在植物体内的积累及其作用[J]. 草业学报, 2023, 32(6): 186-202. |
| [3] | 李超男, 王磊, 周继强, 赵长兴, 谢晓蓉, 刘金荣. 微塑料对紫花苜蓿生长及生理特性的影响[J]. 草业学报, 2023, 32(5): 138-146. |
| [4] | 张振粉, 黄荣, 姚博, 张旺东, 杨成德, 陈秀蓉. 欧美进口紫花苜蓿可培养种带细菌及其对动植物的致病性[J]. 草业学报, 2023, 32(4): 161-172. |
| [5] | 张士敏, 赵娇阳, 朱慧森, 卫凯, 王永新. 硒对不同品种紫花苜蓿发芽阶段物质转化和形态建成的影响[J]. 草业学报, 2023, 32(4): 79-90. |
| [6] | 王园, 王晶, 李淑霞. 紫花苜蓿MsBBX24基因的克隆及耐盐性分析[J]. 草业学报, 2023, 32(3): 107-117. |
| [7] | 田政, 杨正禹, 陆忠杰, 罗奔, 张茂, 董瑞. 44个紫花苜蓿品种的酸铝适应性与耐受性评价[J]. 草业学报, 2023, 32(3): 142-151. |
| [8] | 孙守江, 唐艺涵, 马馼, 李曼莉, 毛培胜. 紫花苜蓿种子吸胀期胚根线粒体AsA-GSH循环对低温胁迫的响应[J]. 草业学报, 2023, 32(3): 152-162. |
| [9] | 刘选帅, 孙延亮, 安晓霞, 马春晖, 张前兵. 施磷和接种解磷菌对紫花苜蓿光合特性及生物量的影响[J]. 草业学报, 2023, 32(3): 189-199. |
| [10] | 王晓龙, 杨曌, 来永才, 李红, 钟鹏, 徐艳霞, 柴华, 李莎莎, 吴玥, 宋敏超, 周景明. 不同秋眠等级苜蓿根系性状对越冬的影响[J]. 草业学报, 2023, 32(1): 144-153. |
| [11] | 孙延亮, 赵俊威, 刘选帅, 李生仪, 马春晖, 王旭哲, 张前兵. 施氮对苜蓿初花期光合日变化、叶片形态及干物质产量的影响[J]. 草业学报, 2022, 31(9): 63-75. |
| [12] | 王星, 黄薇, 余淑艳, 李小云, 高雪芹, 伏兵哲. 宁夏地区地下滴灌水肥耦合对紫花苜蓿种子产量及构成因素的影响[J]. 草业学报, 2022, 31(9): 76-85. |
| [13] | 赵建涛, 岳亚飞, 张前兵, 马春晖. 不同秋眠级紫花苜蓿品种抗寒性对新疆北疆地区覆雪厚度的响应[J]. 草业学报, 2022, 31(8): 24-34. |
| [14] | 刘彩婷, 毛丽萍, 阿依谢木, 于应文, 沈禹颖. 紫花苜蓿与垂穗披碱草混播比例对其抗寒生长生理特征的影响[J]. 草业学报, 2022, 31(7): 133-143. |
| [15] | 王雪萌, 何欣, 张涵, 宋瑞, 毛培胜, 贾善刚. 基于多光谱成像技术快速无损检测紫花苜蓿人工老化种子[J]. 草业学报, 2022, 31(7): 197-208. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||