

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2024, Vol. 33 ›› Issue (6): 155-164.DOI: 10.11686/cyxb2023266
• 研究论文 • 上一篇
郑海1( ), 王莹2, 徐娟1, 朱婷婷1, 秦格格1, 周存宇1, 杨朝东1, 谭德宝2, 张霞1, 魏红波1(
), 王莹2, 徐娟1, 朱婷婷1, 秦格格1, 周存宇1, 杨朝东1, 谭德宝2, 张霞1, 魏红波1( )
)
收稿日期:2023-07-25
修回日期:2023-09-25
出版日期:2024-06-20
发布日期:2024-03-20
通讯作者:
魏红波
作者简介:E-mail: 524392976@qq.com基金资助:
Hai ZHENG1( ), Ying WANG2, Juan XU1, Ting-ting ZHU1, Ge-ge QIN1, Cun-yu ZHOU1, Chao-dong YANG1, De-bao TAN2, Xia ZHANG1, Hong-bo WEI1(
), Ying WANG2, Juan XU1, Ting-ting ZHU1, Ge-ge QIN1, Cun-yu ZHOU1, Chao-dong YANG1, De-bao TAN2, Xia ZHANG1, Hong-bo WEI1( )
)
Received:2023-07-25
Revised:2023-09-25
Online:2024-06-20
Published:2024-03-20
Contact:
Hong-bo WEI
摘要:
以分布在三峡水库消落区和江汉平原河漫滩香附子营养器官为试验材料,采用解剖镜下切片法、组织化学研究方法、光学和荧光显微镜下观察拍照记录试验结果,研究其叶、直立茎、根状茎、块茎和不定根适应两栖环境的解剖结构与组织化学特征。结果表明:1)香附子具有适应两栖环境的气腔和质外体屏障等典型结构特征,其中气腔包含根、根状茎及叶的溶生性通气组织和茎的裂生性通气组织;质外体屏障包括内皮层、外皮层、栓质化中柱和维管束鞘细胞等。2)香附子的不定根和茎具有栓质化中柱和维管束;叶片和茎的花环结构由内侧维管束鞘、中间维管束鞘和薄壁细胞维管束鞘3层组成。3)香附子的质外体屏障可能有助于香附子在淹没缺氧下保持氧气流通,同时控制水、离子与环境交换,气腔有利于保存和输送有氧呼吸所必需的氧气。香附子适应淹没-陆生两栖转换环境的结构特征表明其是长江流域生态恢复的重要植物资源。
郑海, 王莹, 徐娟, 朱婷婷, 秦格格, 周存宇, 杨朝东, 谭德宝, 张霞, 魏红波. 两栖植物香附子的解剖结构和组织化学研究[J]. 草业学报, 2024, 33(6): 155-164.
Hai ZHENG, Ying WANG, Juan XU, Ting-ting ZHU, Ge-ge QIN, Cun-yu ZHOU, Chao-dong YANG, De-bao TAN, Xia ZHANG, Hong-bo WEI. Anatomical and histochemical features of amphibious Cyperus rotundus[J]. Acta Prataculturae Sinica, 2024, 33(6): 155-164.
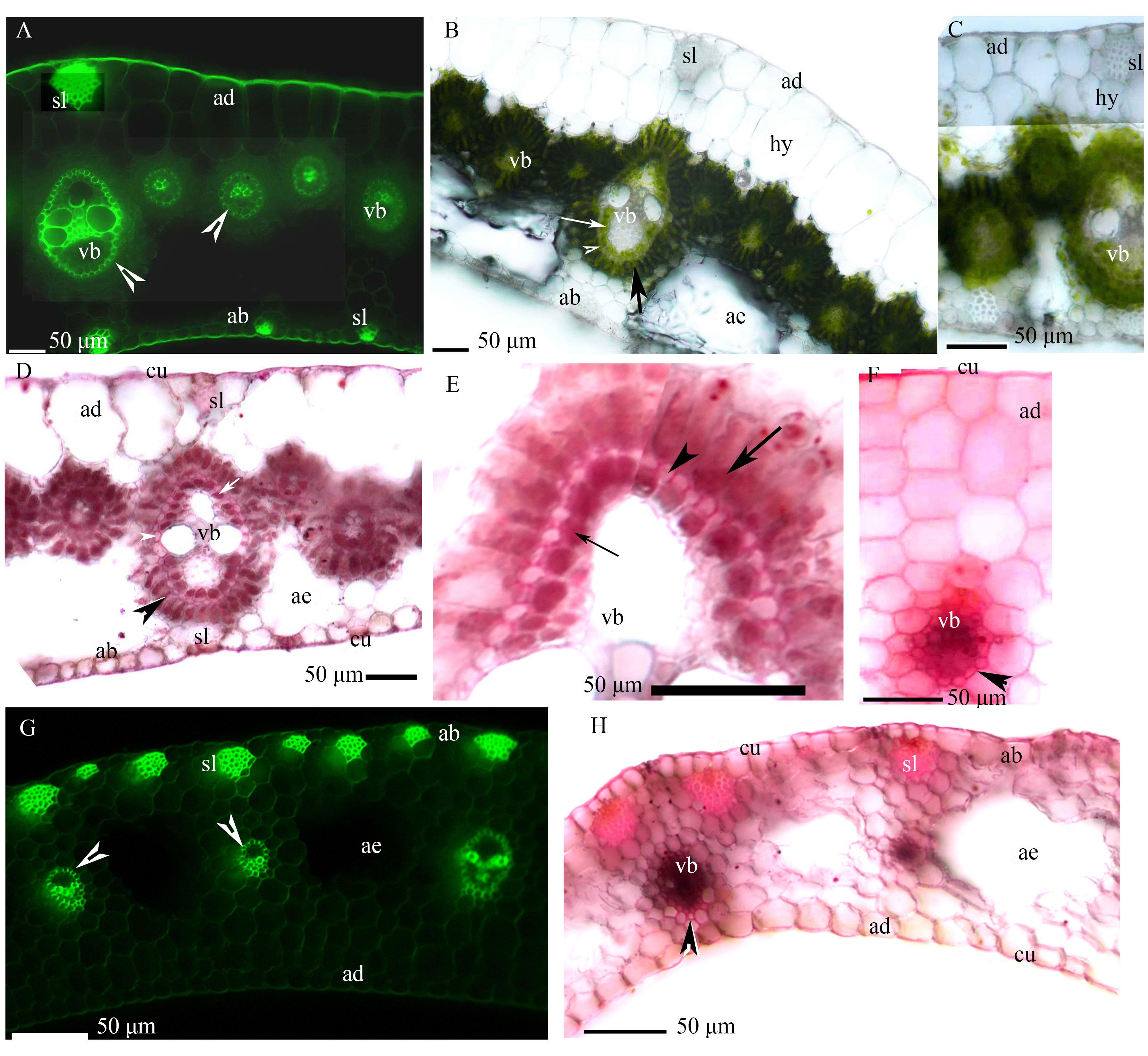
图1 香附子叶片(A~F)和叶鞘(G~H)的显微照片A: 维管束,中间维管束鞘的内皮层具凯氏带(箭头), 近轴面表皮具角质层, 远轴面表皮具角质层、厚壁纤维层,BAB染色; B: 维管束,内侧维管束鞘(细箭头),中间维管束鞘(箭头),外侧薄壁细胞维管束鞘(箭),通气组织,近轴面表皮,远轴面表皮,下表皮,厚壁纤维层,没有染色;C: 维管束,近轴面表皮,皮下层,没有染色; D: 维管束,内侧维管束鞘(细箭头),栓化中间维管束鞘(箭头),薄壁细胞维管束鞘(箭),通气组织,近轴面表皮具角质层,远轴面表皮具角质层、厚壁纤维层,SR7B染色; E: 维管束,内侧维管束鞘(细箭头),栓质化中间维管束鞘(箭头),薄壁细胞维管束鞘(箭),SR7B染色; F: 维管束,栓化中间维管束鞘(箭头),近轴面表皮具角质层,SR7B染色; G: 维管束,维管束鞘具凯氏带(箭头),远轴面表皮,近轴面表皮,通气组织,厚壁纤维层,BAB染色; H: 维管束,栓化维管束鞘(箭头),通气组织,近轴面表皮具角质层,远轴面表皮具角质层、通气组织、厚壁纤维层,SR7B染色。ad: 近轴面表皮; ab: 远轴面表皮; ae: 通气组织; cu: 角质层; hy: 皮下层; sl: 厚壁纤维层; vb: 维管束。下同。A: Vascular bundles, mestome sheaths with casparian strips (endodermis) (arrowhead), adaxial epidermis with cuticle, abaxial epidermis with cuticle, and sclerenchyma fibers. Staining: BAB; B: Vascular bundles, inner sheaths (thin arrow), mestome sheaths (arrowhead), parenchymatous sheaths (arrow), aerenchyma, adaxial epidermis, abaxial epidermis, hypodermis, and sclerenchyma fibers. Unstained; C: Vascular bundles, adaxial epidermis, and hypodermis. Unstained; D: Vascular bundles, inner sheaths (thin arrow), suberized mestome sheaths (arrowhead), parenchymatous sheaths (arrow), aerenchyma, adaxial epidermis with cuticle, abaxial epidermis with cuticle, and sclerenchyma fibers. Staining: SR7B; E: Vascular bundles, inner sheaths (thin arrow), suberized mestome sheaths (arrowhead), and parenchymatous sheaths (arrow). Staining: SR7B; F: Vascular bundles, suberized mestome sheaths (arrowhead), adaxial epidermis with cuticle. Staining: SR7B; G: Vascular bundle, vascular bundle sheaths with casparian strips (arrowhead), abaxial epidermis, adaxial epidermis, aerenchyma, and sclerenchyma fibers. Staining: BAB; H: Vascular bundles, suberized sheaths (arrowhead), aerenchyma, adaxial epidermis with cuticle, abaxial epidermis with cuticle, and sclerenchyma fibers; Staining: SR7B. ad: Adaxial epidermis; ab: Abaxial epidermis; ae: Aerenchyma; cu: Cuticle; hy: Hypodermis; sl: Sclerenchyma fibers; vb: Vascular bundles. The same below.
Fig. 1 Photomicrographs of the leaf blades (A-F), and leaf sheaths (G-H) of C. rotundus
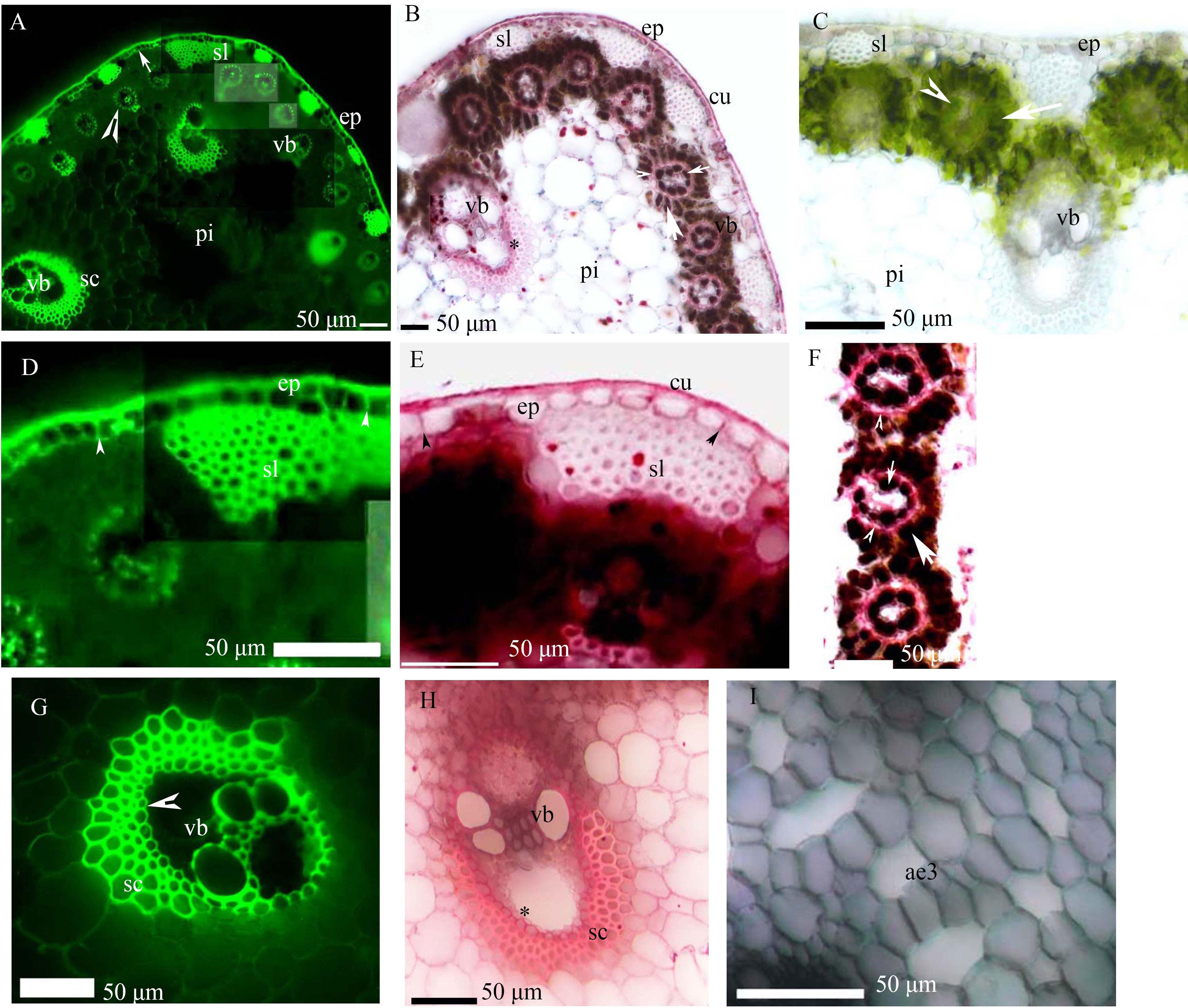
图2 香附子茎的显微照片A: 表皮具单层细胞的外皮层(箭)、厚壁纤维层和维管束, 中间维管束鞘具凯氏带(箭头)、木质化厚壁组织层和髓, BAB染色;B: 表皮外具角质层、厚壁纤维层、维管束、栓质化维管束鞘(*)、内侧维管束鞘(小箭头)、栓质化中间维管束鞘(箭头)、薄壁细胞维管束鞘(箭)和髓, SR7B染色;C: 表皮具厚壁纤维层, 维管束, 内侧维管束鞘(箭头), 薄壁细胞维管束鞘(箭)和髓, 没有染色;D: 图A的局部区域放大, 表皮, 外皮层具凯氏带(箭头), 和厚壁纤维层, BAB染色;E: 表皮外具角质层, 栓质化外皮层(箭头), 厚壁纤维层, SR7B染色;F: 内侧维管束鞘(小箭头), 栓质化中间维管束鞘具(箭头), 薄壁细胞维管束鞘(箭), SR7B染色;G: 维管束鞘具内皮层, 木质化厚壁组织层, BAB染色;H: 栓质化维管束鞘(*)和厚壁组织层, SR7B染色;I: 髓具裂生通气组织, BAB染色, 明场。 ep, 表皮; pi, 髓; sc, 厚壁组织层。下同。A: Epidermis with uniseriate exodermis (arrow), lignified sclerenchyma fibers, vascular bundles, mestome sheaths with casparian strips (arrowhead), lignified sclerenchyma ring, and pith. Staining: BAB; B: Epidermis with cuticle, sclerenchyma fibers, vascular bundles, suberized vascular bundle sheaths (*), inner sheaths (small arrowhead), suberized mestome sheaths (arrowhead), parenchymatous sheaths (arrow), and pith. Staining: SR7B; C: Epidermis, sclerenchyma fibers, vascular bundles, inner sheaths (arrowhead), parenchymatous sheaths (arrow), and pith. Unstained; D: Enlarged area of plate A, epidermis, exodermis with casparian strips (arrowhead), and sclerenchyma fibers. Staining: BAB; E: Epidermis with cuticle, suberized uniseriate exodermis (arrowhead), and sclerenchyma fibers. Staining: SR7B; F: Inner sheaths (small arrow), suberized mestome sheaths (arrowhead), and parenchymatous sheaths (arrow). Staining: SR7B; G: Vascular bundle sheaths with endodermis (arrowhead) and lignified sclerenchyma ring. Staining: BAB; H: Suberized vascular bundle sheaths (*) and sclerenchyma ring. Staining: SR7B; I: Schizogenous aerenchyma in the pith. Staining: BAB under brightfield. ep: Epidermis; pi: Pith; sc: Sclerenchyma ring. The same below.
Fig. 2 Photomicrographs of the C. rotundus culms

图3 香附子根状茎(162~247 mm)的切片A: 髓,维管束,溶生细胞(*),内皮层(箭头),皮层,小维管束鞘具凯氏带(细箭头),单层外皮层(箭),厚壁纤维层,表皮外角质层,BAB染色;B: 髓,维管束,溶生细胞(*),栓质化原生木质部和后生木质部鞘,内皮层(箭头),皮层,通气组织,栓质化维管束鞘(细箭头),单层细胞外皮层(箭),厚壁纤维层,表皮外角质层,插入图展示了单层细胞外皮层(箭)和表皮外角质层,SR7B染色;C: 维管束,皮层,通气组织,表皮,BAB染色明场;D: 同C图切片,髓,维管束,内皮层(箭头),木质化厚壁组织层(*),皮层,小维管束鞘具凯氏带(细箭头),单层细胞外皮层(箭),表皮外角质层,BAB染色;E: D图的局部放大,髓,周木维管束,初生木质部,初生韧皮部,内皮层(箭头),木质化厚壁组织层(*),皮层,小维管束鞘具凯氏带(细箭头),BAB染色,插入图中展示了维管束鞘细胞栓质化(#)和内皮层(箭头),SR7B染色; F: 髓,维管束,栓质化后生木质部导管及维管束鞘细胞,内皮层(箭头),厚壁组织层(*),皮层,通气组织,单层细胞外皮层(箭),表皮外角质层,插入图中展示了单层细胞外皮层(箭),厚壁纤维层和表皮外角质层,SR7B染色。 mx: 后生木质部; pp: 初生韧皮部; xy: 初生木质部; px: 原生木质部; co: 皮层。下同。A: Pith, vascular bundles, lysed cells (*), endodermis (arrowhead), cortex, bundle sheaths with Casparian strips (thin arrow), uniseriate exodermis (arrow), sclerenchyma fibers, and epidermis with cuticle. Staining: BAB; B: Pith, vascular bundles, lysed cells (*), suberized protoxylem and metaxylem poles, endodermis (arrowhead), cortex, aerenchyma, suberized bundle sheaths (thin arrow), uniseriate exodermis (arrow), sclerenchyma fibers, and epidermis with cuticle. Inset shows uniseriate exodermis (arrow), and epidermis with cuticle. Staining: SR7B; C: Vascular bundles, cortex, aerenchyma, and epidermis. Staining: BAB under brightfield; D: Same view as plate C. Pith, vascular bundles, endodermis (arrowhead), lignified sclerenchyma ring (*), cortex, bundle sheaths with casparian strips (thin arrow), uniseriate exodermis (arrow), and epidermis with cuticle. Staining: BAB; E: Enlarged area of plate D. Pith, amphivasal vascular bundles, primary xylem, primary phloem, endodermis (arrowhead), lignified sclerenchyma ring (*), cortex, and bundle sheaths with casparian strips (thin arrow). Staining: BAB. Inset shows suberized pith of central cylinder (#) and endodermis (arrowhead). Staining: SR7B; F: Pith, vascular bundles, suberized metaxylem poles, endodermis (arrowhead), sclerenchyma ring (*), cortex, aerenchyma, uniseriate exodermis (arrow), and epidermis with cuticle. Inset shows uniseriate exodermis (arrow), sclerenchyma fibers, and epidermis with cuticle. Staining: SR7B. mx: Metaxylem; pp: Primary phloem; xy: Primary xylem; px: Protoxylem; co: Cortex. The same below.
Fig. 3 Photomicrographs of C.rotundus rhizomes (162-247 mm long)

图4 香附子块茎的切片A: 具淀粉颗粒的皮层,溶生细胞(*),单层细胞的外皮层(箭),厚壁纤维层,表皮,BAB染色,明场;B: 与A图同一切片,皮层,溶生细胞(*),外皮层(箭),厚壁纤维层,表皮外角质层,插入图展示了外皮层(箭),厚壁纤维层,表皮外角质层,BAB染色;C: 带颗粒的皮层,外皮层(箭),厚壁纤维层,表皮外角质层,插入图展示了外皮层(箭),表皮外角质层,SR7B染色;D: 髓,周木维管束,溶生细胞(*),内皮层(箭头),皮层,BAB染色;E: 髓,维管束,栓质化初生木质部鞘(细箭头),内皮层(箭头),皮层,插入图展示了栓质化初生木质部鞘细胞(细箭头),SR7B染色;F: 带颗粒的皮层,BAB染色,明场。A: Cortex with granules, lysed cells (*), uniseriate exodermis (arrow), sclerenchyma fibers, and epidermis. Staining: BAB under brightfield; B: Same view as plate A. Cortex, lysed cells (*), exodermis (arrow), sclerenchyma fibers, and epidermis with cuticle. Inset shows exodermis (arrow), sclerenchyma fibers, and epidermis with cuticle. Staining: BAB; C: Cortex with granules, exodermis (arrow), sclerenchyma fibers, and epidermis with cuticle. Inset shows exodermis (arrow) and epidermis with cuticle. Staining: SR7B; D: Pith, amphivasal vascular bundles, lysed cells (*), endodermis (arrowhead), and cortex. Staining: BAB; E: Pith, vascular bundles, suberized primary xylem poles (thin arrow), endodermis (arrowhead), and cortex. Inset shows suberized primary xylem poles (arrowhead). Staining: SR7B; F: Cortex with granules. Staining: BAB under brightfield.
Fig. 4 Photomicrographs of the C. rotundus tubers

图5 香附子不定根切片(75~124 mm)A: 原生木质部,内皮层(箭头),木质化厚壁组织层(*),皮层,通气组织,单层细胞外皮层(箭),表皮,BAB染色;B: 原生木质部,内皮层(箭头),皮层,通气组织,单层细胞外皮层(箭),表皮,SR7B染色;C: 原生木质部,后生木质部,内皮层(箭头),木质化厚壁组织层(*),皮层,通气组织,单层细胞外皮层(箭),表皮,BAB染色;D: 栓质化原生木质部,栓质化后生木质部,栓质化中柱,内皮层(箭头),通道细胞,皮层,通气组织,单层细胞外皮层(箭),表皮,SR7B. pc: 通道细胞; st: 中柱; px: 原生木质部; rh: 表皮。A: Protoxylem, endodermis (arrowhead), lignified sclerenchyma ring (*), cortex, aerenchyma, uniseriate exodermis (arrow), and rhizodermis. Staining: BAB; B: Protoxylem, endodermis (arrowhead), cortex, aerenchyma, uniseriate exodermis (arrow), and rhizodermis. Staining: SR7B; C: Protoxylem, metaxylem, endodermis (arrowhead), lignified sclerenchyma ring (*), cortex, aerenchyma, uniseriate exodermis (arrow), and rhizodermis. Staining: BAB; D: Suberized protoxylem poles, suberized metaxylem poles, suberized stele, endodermis (arrowhead), passage cells, cortex, aerenchyma, uniseriate exodermis (arrow), and rhizodermis. Staining: SR7B. pc: passage cells; st: stele; px: protoxylem; rh: rhizodermis.
Fig. 5 Photomicrographs of Cyperus rotundus adventitious roots (75-124 mm long)
| 1 | Pena-Fronteras J T, Villalobos M C, Baltazar A M, et al. Adaptation to flooding in upland and lowland ecotypes of Cyperus rotundus, a troublesome sedge weed of rice: tuber morphology and carbohydrate metabolism. Annals of Botany, 2009, 103(2): 295-302. |
| 2 | Fuentes R G, Baltazar A M, Merca F E, et al. Morphological and physiological responses of lowland purple nutsedge (Cyperus rotundus L.) to flooding. AoB Plants, 2010, plq010: 1-13. |
| 3 | Baloch A H, ur Rehman H, Ibrahim Z, et al. The biology of Balochistani weed: Cyperus rotundus Linnaeus. A Review. Pure and Applied Biology (PAB), 2021, 4(2): 171-180. |
| 4 | Mumtaz S, Hameed M, Ahmad F, et al. Structural and functional modifications in osmoregulation for ecological success in purple nutsedge (Cyperus rotundus). International Journal of Agriculture and Biology, 2019, 22(5): 1123-1132. |
| 5 | Donayre D K M, Jimenez J J L, Latonio A M L S, et al. Lowland ecotype Cyperus rotundus L. affects growth and yield of rice under flooded conditions in the Philippines. BioRxiv, 2021: 2021. 09. 22: 1-25. doi:https://doi.org/10.1101/2021.09.22.461350. |
| 6 | Zhang A, Xie Z. C4 herbs dominate the reservoir flood area of the Three Gorges Reservoir. Science of the Total Environment, 2021, 755: 142479. |
| 7 | Hong M, Guo Q S, Nie B H, et al. Responds of Cyperus rotundus to flooding-drying habitats changes in Three Gorges Reservoir hydro-fluctuation belt. Journal of Agricultural University of Hebei, 2011, 34(3): 77-84. |
| 洪明, 郭泉水, 聂必红, 等. 三峡库区消落带香附子对水陆生境变化的响应. 河北农业大学学报, 2011, 34(3): 77-84. | |
| 8 | Bajpay A, Nainwal R C, Singh D, et al. Medicinal value of Cyperus rotundus Linn: An updated review. Medicinal Plants-International Journal of Phytomedicines and Related Industries, 2018, 10(3): 165-170. |
| 9 | Dor E, Hershenhorn J. Effect of low temperature on purple nutsedge (Cyperus rotundus) reproductive biology. Weed Science, 2013, 61(2): 239-243. |
| 10 | Colmer T D, Gibberd M R, Wiengweera A, et al. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany, 1998, 49(325): 1431-1436. |
| 11 | Enstone D E, Peterson C A, Ma F. Root endodermis and exodermis: Structure, function, and responses to the environment. Journal of Plant Growth Regulation, 2002, 21: 335-351. |
| 12 | Seago Jr J L, Marsh L C, Stevens K J, et al. A re-examination of the root cortex in wetland flowering plants with respect to aerenchyma. Annals of Botany, 2005, 96(4): 565-579. |
| 13 | Seago Jr J L. Anatomy of Wetland Plants//Finlayson C M, Milton R G, Prentice R C (eds). The Wetland Book. Dordrecht: Springer, 2018: 363-374. |
| 14 | Seago Jr J L. Revisiting the occurrence and evidence of endodermis in angiosperm shoots. Flora, 2020, 273: 151709. |
| 15 | Seago Jr J L, Tylová E, Soukup A, et al. A new examination of anatomical structures characterizing the genus Gunnera. Flora, 2021, 283: 151919. |
| 16 | Ranathunge K, Lin J, Steudle E, et al. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant, Cell & Environment, 2011, 34(8): 1223-1240. |
| 17 | Yang C, Zhang X, Zhou C, et al. Root and stem anatomy and histochemistry of four grasses from the Jianghan Floodplain along the Yangtze River, China. Flora-Morphology, Distribution, Functional Ecology of Plants, 2011, 206(7): 653-661. |
| 18 | Yang C, Zhang X, Li J, et al. Anatomy and histochemistry of roots and shoots in wild rice (Zizania latifolia Griseb.). Journal of Botany, 2014: 1-9. https://doi.org/10.1155/2014/181727. |
| 19 | Yang C, Yang X, Zhang X, et al. Anatomical structures of alligator weed (Alternanthera philoxeroides) suggest it is well adapted to the aquatic-terrestrial transition zone. Flora, 2019, 253: 27-34. |
| 20 | Yang C, Zhang X, Wang T, et al. Phenotypic plasticity in the structure of fine adventitious Metasequoia glyptostroboides roots allows adaptation to aquatic and terrestrial environments. Plants, 2019, 8(11): 501. |
| 21 | Pecková E, Tylová E, Soukup A. Tracing root permeability: Comparison of tracer methods. Biologia Plantarum, 2016, 60: 695-705. |
| 22 | Zhang X, Hu L, Yang C, et al. Structural features of Phalaris arundinacea in the Jianghan Floodplain of the Yangtze River, China. Flora, 2017, 229: 100-106. |
| 23 | Zhang X, Yang C, Seago Jr J L. Anatomical and histochemical traits of roots and stems of Artemisia lavandulaefolia and A. selengensis (Asteraceae) in the Jianghan Floodplain, China. Flora, 2018, 239: 87-97. |
| 24 | Soukup A, Tylová E. Apoplastic barriers: Their structure and function from a historical perspective. Concepts in Cell Biology-History and Evolution, 2018, 23: 155-183. |
| 25 | Mertz R A, Brutnell T P. Bundle sheath suberization in grass leaves: Multiple barriers to characterization. Journal of Experimental Botany, 2014, 65(13): 3371-3380. |
| 26 | Wigoda N, Moshelion M, Moran N. Is the leaf bundle sheath a “smart flux valve” for K+ nutrition. Journal of Plant Physiology, 2014, 171(9): 715-722. |
| 27 | Crang R, Lyons-Sobaski S, Wise R. Plant anatomy: A concept-based approach to the structure of seed plants. Switzerland: Springer Cham, 2019. |
| 28 | Danila F R, Thakur V, Chatterjee J, et al. Bundle sheath suberisation is required for C4 photosynthesis in a Setaria viridis mutant. Communications Biology, 2021, 4(1): 254. |
| 29 | Takahashi H, Yamauchi T, Colmer T D, et al. Aerenchyma formation in plants. Low-Oxygen Stress in Plants: Oxygen Sensing and Adaptive Responses to Hypoxia, 2014: 247-265. |
| 30 | Zhou C, Zhang X, Guo Y, et al. Structural and histochemical analyses of the vegetative organs of Eichhornia crassipes. Botany Letters, 2021, 168(3): 458-466. |
| 31 | Peterson R L, Peterson C A, Peterson C A, et al. Teaching plant anatomy through creative laboratory exercises. Ottawa, Ontario: NRC Research Press, 2008. |
| 32 | Martins S, Scatena V L. Anatomical variations in scapes of Eleocharis minima Kunth (Cyperaceae, Poales)-amphibian and Kranz species. Rodriguésia, 2015, 66(2): 627-631. |
| 33 | Yang C D, Li S F, Yao L, et al. A study of anatomical structure and apoplastic barrier characteristics of Hydrocotyle sibthorpioides. Acta Prataculturae Sinica, 2015, 24(7): 139-145. |
| 杨朝东, 李守峰, 姚兰, 等. 天胡荽的解剖和屏障结构特征研究. 草业学报, 2015, 24(7): 139-145. | |
| 34 | Xiang J, Ming J, Yin H, et al. Anatomy and histochemistry of the roots and shoots in the aquatic selenium hyperaccumulator Cardamine hupingshanensis (Brassicaceae). Open Life Sciences, 2019, 14(1): 318-326. |
| 35 | Šottníková A, Lux A. Development, dilation and subdivision of cortical layers of gentian (Gentiana asclepiadea) root. New Phytologist, 2003, 160(1): 135-143. |
| 36 | Evert R F. Esau’s plant anatomy: Meristems, cells, and tissues of the plant body: Their structure, function, and development. New Jersey: John Wiley & Sons, Inc, 2006. |
| 37 | Ruzin S E. Plant microtechnique and microscopy. New York: Oxford University Press, 1999. |
| 38 | Brundrett M C, Enstone D E, Peterson C A. A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma, 1988, 146: 133-142. |
| 39 | Seago Jr J L, Peterson C A, Enstone D E, et al. Development of the endodermis and hypodermis of Typha glauca Godr. and Typha angustifolia L. roots. Canadian Journal of Botany, 1999, 77(1): 122-134. |
| 40 | Lundgren M R, Osborne C P, Christin P A. Deconstructing Kranz anatomy to understand C4 evolution. Journal of Experimental Botany, 2014, 65(13): 3357-3369. |
| 41 | Fahn A. Plant anatomy, 4th edition. Oxford: Pergamon Press, 1990. |
| 42 | Yang C, Zhang X, Seago Jr J L, et al. Anatomical and histochemical features of Brasenia schreberi (Cabombaceae) shoots. Flora, 2020, 263: 151524. |
| 43 | Wu D, Li L, Ma X, et al. Morphological and anatomical adaptations to dry, shady environments in Adiantum reniforme var. sinense (Pteridaceae). Peer J, 2020, 8: e9937. |
| 44 | Vecchia F D, Cuccato F, Rocca N L, et al. Endodermis-like sheaths in the submerged freshwater macrophyte Ranunculus trichophyllus Chaix. Annals of Botany, 1999, 83(1): 93-97. |
| 45 | McManus H A, Seago Jr J L, Marsh L C. Epifluorescent and histochemical aspects of shoot anatomy of Typha latifolia L., Typha angustifolia L. and Typha glauca Godr. Annals of Botany, 2002, 90(4): 489-493. |
| 46 | Soukup A, Armstrong W, Schreiber L, et al. Apoplastic barriers to radial oxygen loss and solute penetration: A chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist, 2007, 173(2): 264-278. |
| 47 | Meyer C J, Seago Jr J L, Peterson C A. Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Annals of Botany, 2009, 103(5): 687-702. |
| 48 | Bailey-Serres J, Voesenek L. Flooding stress: Acclimations and genetic diversity. Annual Review of Plant Biology, 2008, 59: 313-339. |
| 49 | Kitin P, Nakaba S, Hunt C G, et al. Direct fluorescence imaging of lignocellulosic and suberized cell walls in roots and stems. AoB Plants, 2020, 12(4): plaa032. |
| [1] | 姚瑞瑞, 刘欢, 赵桂琴, 王敬龙, 王绮玉, 董凯, 张然. 贮藏时间对皮、裸燕麦种子萌发及细胞学结构的影响[J]. 草业学报, 2024, 33(2): 154-163. |
| [2] | 谭炯锐, 查同刚, 张泽宇, 张晓霞, 滕红梅, 王玲丽, 赵莉丽, 王奥, 王馨珧. 猪毛菜响应干旱胁迫的叶片结构、生理及转录组分析[J]. 草业学报, 2024, 33(1): 75-88. |
| [3] | 钱文武, 郭鹏, 朱慧森, 张士敏, 李德颖. 草地早熟禾叶片表皮特征、解剖结构及光合特性对不同施氮量的响应[J]. 草业学报, 2023, 32(1): 131-143. |
| [4] | 董梦宇, 王金鑫, 吴萌, 周子瑶, 程顺, 李彦慧. 两种香花芥属植物叶片结构及光合特性研究[J]. 草业学报, 2022, 31(7): 172-184. |
| [5] | 王贞升, 李彦雪, 于成龙, 狄小琳, 陈鹏, 田静瑶, 王竞红. 不同模拟降水量下草地早熟禾根系形态与解剖结构的动态变化[J]. 草业学报, 2020, 29(10): 70-80. |
| [6] | 陈斌, 李洪瑶, 刘筱玮, 夏斌, 孙绍文, 孙颖, 何淼. 不同光照强度对新娘草叶片形态建成及超微结构的影响[J]. 草业学报, 2019, 28(7): 175-185. |
| [7] | 张翠梅, 师尚礼, 刘珍, 杨帆, 张振科. 干旱胁迫对不同抗旱性苜蓿品种根系形态及解剖结构的影响[J]. 草业学报, 2019, 28(5): 79-89. |
| [8] | 王晓娥, 张梵, 张霞, 周存宇, 杨朝东. 虉草适应湿地环境的解剖结构和组织化学特征研究[J]. 草业学报, 2019, 28(1): 86-94. |
| [9] | 张咏梅, 马晖玲, 唐云智. 紫花苜蓿叶片受白粉病菌侵染后结构的变化[J]. 草业学报, 2017, 26(2): 88-94. |
| [10] | 张咏梅, 马晖玲, 唐云智. 豌豆白粉菌侵染对活性氧迸发规律和紫花苜蓿叶片结构的影响[J]. 草业学报, 2016, 25(11): 34-42. |
| [11] | 杨朝东, 李守峰, 姚兰, 艾训儒, 蔡小东, 张霞. 天胡荽的解剖和屏障结构特征研究[J]. 草业学报, 2015, 24(7): 139-145. |
| [12] | 杨朝东,李守峰,邓仕明,姚兰,袁龙义,张霞. 白茅解剖结构和屏障结构特征研究[J]. 草业学报, 2015, 24(3): 213-218. |
| [13] | 程薪宇,刘玫,张欣欣,王臣,李滨胜. 东北毛茛科植物营养器官结构及其系统学意义[J]. 草业学报, 2014, 23(3): 62-74. |
| [14] | 任爱琴,易津,高洪文,李俊,王学敏. 柠条锦鸡儿CkNCED1基因启动子的克隆及表达分析[J]. 草业学报, 2013, 22(2): 165-170. |
| [15] | 王桂芹,高瑞如,王玉良,柴瑞娟. 异质生境空心莲子草的结构基础与生态适应性[J]. 草业学报, 2011, 20(4): 143-152. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||