

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2023, Vol. 32 ›› Issue (2): 178-190.DOI: 10.11686/cyxb2022078
• 综合评述 • 上一篇
收稿日期:2022-02-18
修回日期:2022-03-28
出版日期:2023-02-20
发布日期:2022-12-01
通讯作者:
伍国强
作者简介:E-mail: wugq08@126.com基金资助:
Pan-pan REN( ), Guo-qiang WU(
), Guo-qiang WU( ), Ming WEI
), Ming WEI
Received:2022-02-18
Revised:2022-03-28
Online:2023-02-20
Published:2022-12-01
Contact:
Guo-qiang WU
摘要:
维持K+稳态是植物抵御非生物胁迫的重要策略之一。外向整流K+通道(stelar K+ outward rectifier, SKOR)是一类定位于植物根中柱细胞质膜的 K+外向整流通道。该通道介导细胞质 K+外流以及木质部 K+装载,一方面可以调节细胞内 K+稳态,另一方面通过控制木质部 K+流以维持植物根部和地上部的 K+平衡,在植物响应盐胁迫、干旱胁迫、营养胁迫等过程中起着决定性作用。因此,对 SKOR功能、调控的研究及其应用对作物生产和抗逆性增强有着重要的意义。对 SKOR的发现、结构与分类、表达调控、生物学功能及其非生物胁迫响应等方面的研究成果加以综述,并对该通道未来研究方向进行了展望,以期为农作物品质和抗逆性的遗传改良提供理论依据和基因资源。
任盼盼, 伍国强, 魏明. 植物外向整流K+通道SKOR研究进展[J]. 草业学报, 2023, 32(2): 178-190.
Pan-pan REN, Guo-qiang WU, Ming WEI. Research progress relating to stelar K+ outward rectifier (SKOR) channels in plants[J]. Acta Prataculturae Sinica, 2023, 32(2): 178-190.
物种 Species | 基因名称 Gene name | 登录号 Accession No. | 氨基酸数目 Amino acids (aa) | 表达部位 Expression site | 参考文献 Reference |
|---|---|---|---|---|---|
| 拟南芥 A.thaliana | AtSKOR | AJ223357 | 828 | 根Root | [ |
| 小花茅碱 P. tenuiflora | PtSKOR | JQ279059.1 | 715 | 根Root | [ |
| 水稻 O. sativa | OsK5.1 | Os04g36740 | 719 | 根维管束组织Root vascular tissues | [ |
| OsK5.2 | Os06g14030 | 858 | 根和地上部Root and shoot | [ | |
| 霸王Z. xanthoxylum | ZxSKOR | / | 847 | 根、茎、叶Root, stem and leaf | [ |
| 枸杞L. barbarum | LbSKOR | KU523244 | 815 | 根、叶Root, leaf | [ |
| 甜瓜C. melo | CmSKOR | MF447462 | 825 | 根、茎、叶Root, stem and leaf | [ |
| 烟草Nicotiana tabacum | NtSKOR | XM_009764356.1 | 827 | 主要在根Mainly in root | [ |
| 黑果枸杞Lycium ruthenicum | LrSKOR | KY563342 | 815 | 根、叶Root, leaf | [ |
| 葡萄Vitis vinifera | VvK5.1 | XP_010660282.1 | 821 | 根中柱、侧根Root stelar, lateral root | [ |
| 蒺藜苜蓿Medicago truncatula | MtSKOR | Medtr5g077770 | 835 | 根和地上部Root and shoot | [ |
| 长穗偃麦草Elytrigia elongata | EeSKOR | MK203848 | 717 | 根、叶和叶柄Root, leaves and petioles | [ |
| 灰楸Catalpa fargesii | CfSKOR | MW773724 | 632 | 根Root | [ |
| 山新杨Populus davidiana | PdSKOR | MT335814 | 841 | 根Root | [ |
| 小米Setaria italica | SiSKOR | AT3G02850 | 854 | 根Root | [ |
| 紫柳Salix purpurea | SpuSKOR | SapurV1A.0223s0270 | 1032 | 根、叶、雌蕊、花瓣Root, leaf, pistil and petal | [ |
| 木豆Cajanus cajan | CcSKOR | XP 020202567 | 837 | 根Root | [ |
| 绿豆Vigna radiata | VrSKOR | XP_014505028.1 | 851 | 根Root | [ |
| 甜菜Beta vulgaris | BvSKOR | Bv2_047040_fjjf | 828 | 根和地上部Root and shoot | 未发表数据 Unpublished data |
表1 不同植物SKOR基因
Table 1 The SKOR genes in different plants
物种 Species | 基因名称 Gene name | 登录号 Accession No. | 氨基酸数目 Amino acids (aa) | 表达部位 Expression site | 参考文献 Reference |
|---|---|---|---|---|---|
| 拟南芥 A.thaliana | AtSKOR | AJ223357 | 828 | 根Root | [ |
| 小花茅碱 P. tenuiflora | PtSKOR | JQ279059.1 | 715 | 根Root | [ |
| 水稻 O. sativa | OsK5.1 | Os04g36740 | 719 | 根维管束组织Root vascular tissues | [ |
| OsK5.2 | Os06g14030 | 858 | 根和地上部Root and shoot | [ | |
| 霸王Z. xanthoxylum | ZxSKOR | / | 847 | 根、茎、叶Root, stem and leaf | [ |
| 枸杞L. barbarum | LbSKOR | KU523244 | 815 | 根、叶Root, leaf | [ |
| 甜瓜C. melo | CmSKOR | MF447462 | 825 | 根、茎、叶Root, stem and leaf | [ |
| 烟草Nicotiana tabacum | NtSKOR | XM_009764356.1 | 827 | 主要在根Mainly in root | [ |
| 黑果枸杞Lycium ruthenicum | LrSKOR | KY563342 | 815 | 根、叶Root, leaf | [ |
| 葡萄Vitis vinifera | VvK5.1 | XP_010660282.1 | 821 | 根中柱、侧根Root stelar, lateral root | [ |
| 蒺藜苜蓿Medicago truncatula | MtSKOR | Medtr5g077770 | 835 | 根和地上部Root and shoot | [ |
| 长穗偃麦草Elytrigia elongata | EeSKOR | MK203848 | 717 | 根、叶和叶柄Root, leaves and petioles | [ |
| 灰楸Catalpa fargesii | CfSKOR | MW773724 | 632 | 根Root | [ |
| 山新杨Populus davidiana | PdSKOR | MT335814 | 841 | 根Root | [ |
| 小米Setaria italica | SiSKOR | AT3G02850 | 854 | 根Root | [ |
| 紫柳Salix purpurea | SpuSKOR | SapurV1A.0223s0270 | 1032 | 根、叶、雌蕊、花瓣Root, leaf, pistil and petal | [ |
| 木豆Cajanus cajan | CcSKOR | XP 020202567 | 837 | 根Root | [ |
| 绿豆Vigna radiata | VrSKOR | XP_014505028.1 | 851 | 根Root | [ |
| 甜菜Beta vulgaris | BvSKOR | Bv2_047040_fjjf | 828 | 根和地上部Root and shoot | 未发表数据 Unpublished data |
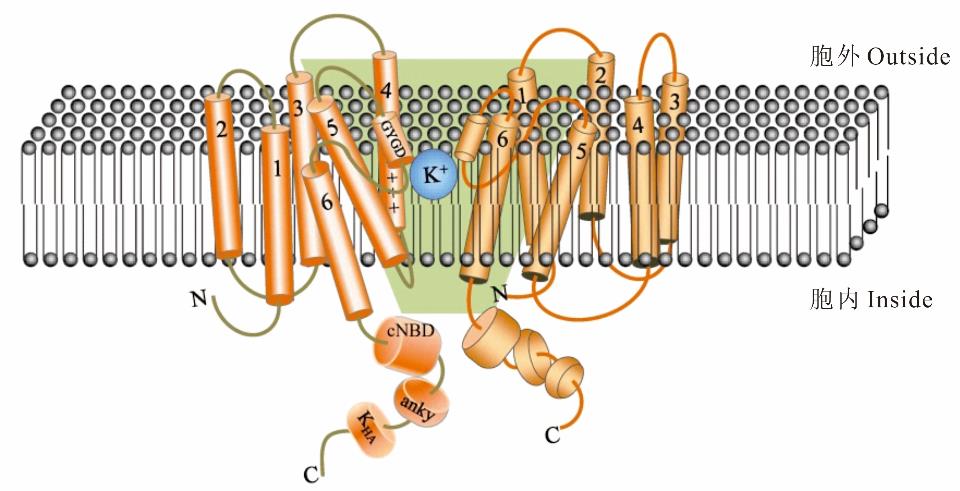
图1 SKOR跨膜结构及组装方式模型图所示为SKOR通道的横截面。橙色部分为两个独立的SKOR α亚基,1~6为跨膜α螺旋。绿色背景范围为中心孔道,由相邻α亚基的S5、S6倾斜相夹而成,S5和S6之间有P环,包含高度保守的序列GYGD,可与K+配位,是通道离子选择性的关键。前4个α-螺旋位于中心孔的外围,其中S4富含带正电荷的氨基酸残基,可以感受到跨膜电压差。N、C表示亚基的N-末端、C-末端,C-末端有cNBD、anky、KHA结构域。A cross section of the SKOR channel is shown above. Orange part represent two separate α subunits, and 1-6 are transmembrane α helices, respectively. The range shown on the green background is the central channel, which is formed by the oblique phase of S5 and S6 of the adjacent α subunit. There is a P loop between S5 and S6, containing the highly conserved sequence GYGD, which can form complex compound with K+, and constitute the key to the ion selectivity of the channel. The S1-4 α-helix surrounds the central hole. S4 is rich in positively charged amino acid residues and can sense transmembrane voltage difference. N and C represent the N-terminal and C-terminal of the subunit, and the C-terminal has cNBD, anky and KHA domains.
Fig.1 Transmembrane structure and assembly method of SKOR[48,54,57]
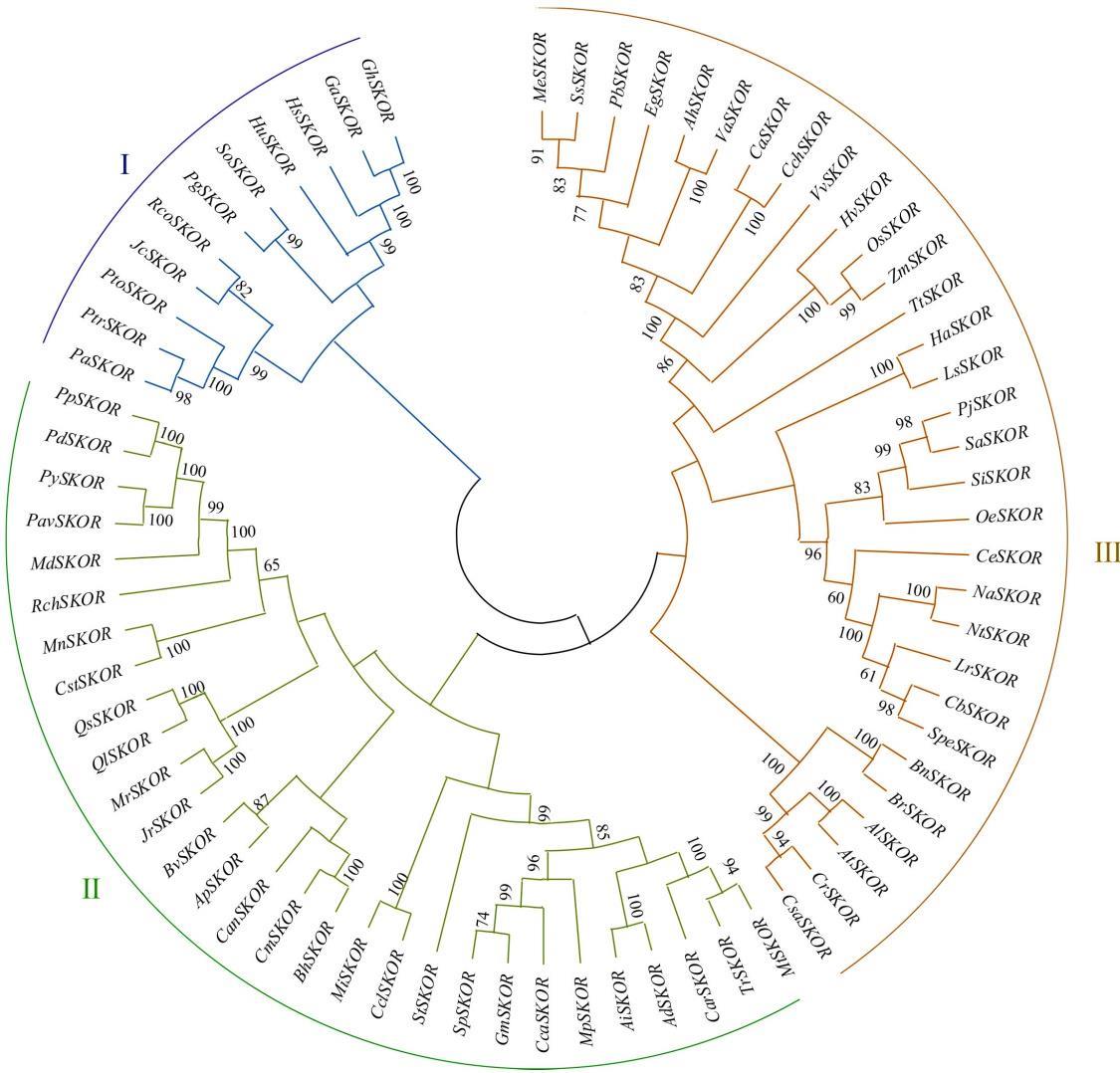
图2 不同物种SKOR系统发育分析分支点数值代表bootstrap分值。SKOR基因的来源、名称及蛋白登录号如下。The branch value represents the bootstrap score. The source, name and protein registration number of SKOR gene are shown below:空心莲子草A. philoxeroidesApSKOR(AFO70199.1);落花生Arachis hypogaeaAhSKOR(QHO59748.1);花生Arachis ipaensisAiSKOR(XP_016166648.1);蔓花生Arachis duranensisAdSKOR(XP_015931612.1);玉山筷子芥ArabidopsislyrataAlSKOR(XP_020889326.1);拟南芥A. thalianaAtSKOR(CAA11280.1); 甘蓝油菜BrassicanapusBnSKOR(XP_013735733.1);芜菁BrassicarapaBrSKOR(XP_009134714.1);冬瓜BenincasahispidaBhSKOR(XP_038876195.1);亚麻荠Camelina sativa CsaSKOR(XP_010485613.1);荠菜CapsellarubellaCrSKOR(XP_006296988.1);大麻Cannabissativa CstSKOR(XP_030505721.1);长辣椒CapsicumannuumCanSKOR(KAF3672329.1);风铃辣椒CapsicumbaccatuCbSKOR(PHT31135.1);中华辣椒CapsicumchinenseCchSKOR(PHU23454.1);木豆CajanuscajanCcaSKOR(XP_020202567.1);香樱桃咖啡CoffeaeugenioidesCeSKOR(XP_027178269.1);甜瓜C.meloCmSKOR(AXL94152.1);南瓜CucurbitaargyrospermaCaSKOR(KAG7032738.1);鹰嘴豆CicerarietinumCarSKOR(XP_004490839.1);克莱门柚Citrusclementina CclSKOR(XP_006427880.1);巨桉EucalyptusgrandisEgSKOR(XP_018717459.2);大豆GlycinemaxGmSKOR(XP_003544361.1); 澳洲棉GossypiumaustraleGaSKOR(KAA3489477.1);陆地棉GossypiumhirsutumGhSKOR(XP_040969227.1);木槿HibiscussyriacusHsSKOR(KAE8656882.1);大麦H.vulgareHvSKOR(KAE8813002.1);向日葵HelianthusannuusHaSKOR(XP_022016115.1);哥伦比亚锦葵HerraniaumbraticaHuSKOR(XP_021298261.1);麻风树JatrophacurcasJcSKOR(XP_037493385.1);胡桃JuglansregiaJrSKOR(XP_035545242.1);莴苣LactucasativaLsSKOR(XP_023735573.1);苹果MalusdomesticaMdSKOR(XP_028964436.1);芒果MangiferaindicaMiSKOR(XP_044506365.1);木薯ManihotesculentaMeSKOR(XP_021615874.1);刺毛黧豆MucunapruriensMpSKOR(RDY07502.1);杨梅MorellarubraMrSKOR(KAB1211342.1);川桑MorusnotabilisMnSKOR(EXC20599.1);野生烟草NicotianaattenuataNaSKOR(XP_019223519.1);茸毛烟草NicotianatomentosiformisNtSKOR(XP_009626879.1);油橄榄OleaeuropaeaOeSKOR(CAA2973405.1);甜樱桃PrunusaviumPavSKOR(XP_021830945.1); 扁桃PrunusdulcisPdSKOR(XP_034209749.1);毛桃PrunuspersicaPpSKOR(XP_007217689.1);石榴PunicagranatumPgSKOR(XP_031396704.1);松蒿PhtheirospermumjaponicumPjSKOR(GFP94887.1);银白杨PopulusalbaPaSKOR(XP_034919423.1);毛白杨PopulustomentosaPtoSKOR(AXY97650.1);毛果杨PopulustrichocarpaPtrSKOR(XP_006372521.1);杜梨PyrusbetulifoliaPbSKOR(AKI29088.1);东京樱花PrunusyedoensisPySKOR(PQM40740.1);白栎QuercuslobataQlSKOR(XP_030952917.1);软木橡树QuercussuberQsSKOR(XP_023922009.1);蓖麻RicinuscommunisRcoSKOR(XP_002533481.2);月季RosachinensisRchSKOR(XP_024187428.1);鸡血藤SpatholobussuberectusSsSKOR(TKY56682.1);地脚金StrigaasiaticaSaSKOR(GER27272.1); 澳洲赤楠SyzygiumoleosumSoSKOR(XP_030461195.1);芝麻SesamumindicumSiSKOR(XP_020552116.1);决明SennatoraStSKOR(KAF7831133.1); 潘那利番茄Solanumpennellii SpeSKOR(XP_015057293.1);白车轴草TrifoliumrepensTrSKOR(AWS33578.1);乌拉尔图小麦T. urartuTuSKOR(EMS58765.1);日本赤豆VignaangularisVaSKOR(KAG2411190.1);葡萄V.viniferaVvSKOR(XP_002279184.2);玉米ZeamaysZmSKOR(AQK83498.1)。其他物种、基因名称和登录号见表1。Other species, gene names and accession numbers are shown in Table 1.
Fig.2 Phylogenetic analysis of SKOR in different species
| 1 | Kumar P, Kumar T, Singh S, et al. Potassium: A key modulator for cell homeostasis. Journal of Biotechnology, 2021, 38(324): 198-210. |
| 2 | Huang Y, Guan C, Liu Y, et al. Enhanced growth performance and salinity tolerance in transgenic switchgrass via overexpressing vacuolar Na+(K+)/H+ antiporter gene (PvNHX1). Frontiers in Plant Science, 2017, 8: 458. |
| 3 | Pathak J, Ahmed H, Kumari N, et al. Role of calcium and potassium in amelioration of environmental stress in plants// Roychoudhury A, Tripathi D K. Protective chemical agents in the amelioration of plant abiotic stress (First Edition). Manhattan: Biochemical and Molecular Perspectives, 2020: 535-562. |
| 4 | Luan M, Tang R J, Tang Y, et al. Transport and homeostasis of potassium and phosphate: Limiting factors for sustainable crop production. Journal of Experimental Botany, 2017, 68(12): 3091-3105. |
| 5 | Véry A A, Nieves-Cordones M, Daly M, et al. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species. Journal of Plant Physiology, 2014, 171(9): 748-769. |
| 6 | Ragel P, Raddatz N, Leidi E O, et al. Regulation of K+ nutrition in plants. Frontiers in Plant Science, 2019, 10: 281. |
| 7 | Nieves-Cordones M, Alemán F, Martínez V, et al. K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. Journal of Plant Physiology, 2014, 171(9): 688-695. |
| 8 | Santa-María G E, Oliferuk S, Moriconi J I. KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: A twenty years tale. Journal of Plant Physiology, 2018, 226(4): 77-90. |
| 9 | Villette J, Cuéllar T, Zimmermann S D, et al. Unique features of the grapevine VvK5.1 channel support novel functions for outward K+ channels in plants. Journal of Experimental Botany, 2019, 70(21): 6181-6193. |
| 10 | Pilot G, Pratelli R, Gaymard F, et al. Five-group distribution of the shaker-like K+ channel family in higher plants. Journal of Molecular Evolution, 2003, 56(4): 418-434. |
| 11 | Hirsch R E, Lewis B D, Spalding E P, et al. A role for the AKT1 potassium channel in plant nutrition. Science, 1998, 280(5365): 918-921. |
| 12 | Mouline K, Véry A A, Gaymard F, et al. Pollen tube development and competitive ability are impaired by disruption of a shaker K+ channel in Arabidopsis. Genes & Development, 2002, 16(3): 339-350. |
| 13 | Anderson J A, Huprikar S S, Kochian L V, et al. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(9): 3736-3740. |
| 14 | Schachtman D P, Schroeder J I, Lucas W J, et al. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science, 1992, 258(5088): 1654-1658. |
| 15 | Pilot G, Lacombe B T, Gaymard F, et al. Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. Journal of Biological Chemisitry, 2001, 276(5): 3215-3221. |
| 16 | Dreyer I, Michard E, Lacombe B T, et al. A plant shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or ‘leak’current. FEBS Letters, 2001, 505(2): 233-239. |
| 17 | Xicluna J, Lacombe B, Dreyer I, et al. Increased functional diversity of plant K+ channels by preferential heteromerization of the shaker-like subunits AKT2 and KAT2. The Journal of Biological Chemistry, 2007, 282(1): 486-494. |
| 18 | Dreyer I, Antunes S, Hoshi T, et al. Plant K+ channel alpha-subunits assemble indiscriminately. Biophysical Journal, 1997, 72(5): 2143-2150. |
| 19 | Reintanz B, Szyroki A, Ivashikina N, et al. AtKC1, a silent Arabidopsis potassium channel α-subunit modulates root hair K+ influx. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(6): 4079-4084. |
| 20 | Duby G, Hosy E, Fizames C, et al. AtKC1, a conditionally targeted shaker-type subunit, regulates the activity of plant K+ channels. The Plant Journal, 2008, 53(1): 115-123. |
| 21 | Gaymard F, Pilot G, Lacombe B, et al. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell, 1998, 94(5): 647-655. |
| 22 | Ache P, Becker D, Ivashikina N, et al. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Letters, 2000, 486(2): 93-98. |
| 23 | Johansson I, Wulfetange K, Porée F, et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant Journal, 2006, 46(2): 269-281. |
| 24 | Liu K, Li L, Luan S. Intracellular K+ sensing of SKOR, a shaker-type K+ channel from Arabidopsis. The Plant Journal, 2006, 46(2): 260-268. |
| 25 | Wegner L H, Raschke K. Ion channels in the xylem parenchyma of barley roots a procedure to isolate protoplasts from this tissue and a patch-clamp exploration of salt passageways into xylem vessels. Plant Physiology, 1994, 105(3): 799-813. |
| 26 | Roberts S K, Tester M. Inward and outward K+-selective currents in the plasma membrane of protoplasts from maize root cortex and stele. The Plant Journal, 1995, 8(6): 811-825. |
| 27 | Duan L J. Cloning, construction RNAi vector of PtSKOR gene and expression pattern analysis of salt-tolerant genes in Puccinellia tenuiflora. Lanzhou: Lanzhou University, 2015. |
| 段丽婕. 小花碱茅PtSKOR基因的克隆、RNAi载体的构建及耐盐相关基因的表达分析. 兰州: 兰州大学, 2015. | |
| 28 | Kim H Y, Choi E H, Min M K, et al. Differential gene expression of two outward-rectifying shaker-like potassium channels OsSKOR and OsGORK in rice. Journal of Plant Biology, 2015, 58(4): 230-235. |
| 29 | Hu J, Ma Q, Kumar T, et al. ZxSKOR is important for salinity and drought tolerance of Zygophyllum xanthoxylum by maintaining K+ homeostasis. Plant Growth Regulation, 2016, 80(2): 195-205. |
| 30 | Zhang H, Wei S, Hu W, et al. Arbuscular mycorrhizal fungus Rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Frontiers in Plant Science, 2017, 8: 440. |
| 31 | Huang L T, Zhao L N, Guo L W, et al. Constitutive expression of CmSKOR, an outward K+ channel gene from melon, in Arabidopsis thaliana involved in saline tolerance. Plant Science, 2018, 274(9): 492-502. |
| 32 | Nguyen T H, Huang S, Meynard D, et al. A dual role for the OsK5.2 ion channel in stomatal movements and K+ loading into xylem sap. Plant Physiology, 2017, 174(4): 2409-2418. |
| 33 | Zhuo W, Chen Q, Yang S Y, et al. Cloning and expression analysis of potassium channel NtSKOR gene in Nicotiana tabacum. Acta Agriculturae Boreali-Sinica, 2018, 33(5): 99-105. |
| 卓维, 陈倩, 杨尚谕, 等. 烟草K+通道NtSKOR基因的克隆及表达分析.华北农学报, 2018, 33(5): 99-105. | |
| 34 | Liu L P, Dai F B, Zhang C, et al. Cloning and expression analysis of the SKOR gene for an outward rectifying K+ channel in Lycium ruthenicum. Journal of Zhejiang A & F University, 2018, 35(1): 104-111. |
| 刘丽萍, 戴逢斌, 张冲, 等. 黑果枸杞外整流钾离子通道SKOR基因的克隆及表达分析. 浙江农林大学学报, 2018, 35(1): 104-111. | |
| 35 | Drain A, Thouin J, Wang L, et al. Functional characterization and physiological roles of the single shaker outward K+ channel in Medicago truncatula. The Plant Journal, 2020, 102(6): 1249-1265. |
| 36 | Zhang Y, Tian X X, Zheng M L, et al. Cloning and functional analysis of the EeSKOR promoter of Elytrigia elongata. Acta Botanica Boreali-Occidentalia Sinica, 2021, 41(11): 1810-1817. |
| 张勇, 田小霞, 郑明利, 等. 长穗偃麦草EeSKOR启动子的克隆及功能分析. 西北植物学报, 2021, 41(11): 1810-1817. | |
| 37 | Fei Y, Zhang Y, Hu J W, et al. Cloning and expression analysis of the CfSKOR gene for anoutward rectifying K+ channel in Catalpa fargesii. Molecular Plant Breeding, 2021, 19(13): 4350-4361. |
| 费越, 张玉, 胡继文, 等. 灰楸外整流钾离子通道CfSKOR基因的克隆及表达分析.分子植物育种, 2021, 19(13): 4350-4361. | |
| 38 | Wang L M, Chen Y H, Yang Q S, et al. Cloning and functional analysis of potassium channel gene PdbSKOR in Populus davidiana×P. bolleana. Scientia Silvae Sinicae, 2021, 57(1): 53-63. |
| 王力敏, 陈亚辉, 杨庆山, 等. 山新杨钾离子通道基因PdbSKOR的克隆与功能分析.林业科学, 2021, 57(1): 53-63. | |
| 39 | Zhang B, Wang H, Guo Y, et al. Identification and characterization of shaker K+ channel gene family in foxtail millet (Setaria italica) and their role in stress response. Front in Plant Science. 2022, 9(13): 907635. |
| 40 | Chen Y, Peng X, Cui J, et al. Isolation and functional determination of SKOR potassium channel in purple osier willow, Salix purpurea. International Journal of Genomics, 2021, 2021: 6669509. |
| 41 | Siddique M H, Babar N I, Zameer R, et al. Genome-wide identification, genomic organization, and characterization of potassium transport-related genes in Cajanus cajan and their role in abiotic stress. Plants, 2021, 10(11): 2238. |
| 42 | Azeem F, Ijaz U, Ali M A, et al. Genome-wide identification and expression profiling of potassium transport-related genes in Vigna radiata under abiotic stresses. Plants, 2022, 11(1): 2. |
| 43 | Porée F, Wulfetange K, Naso A, et al. Plant Kin and Kout channels: Approaching the trait of opposite rectification by analyzing more than 250 KAT1-SKOR chimeras. Biochemical and Biophysical Research Communications, 2005, 332(2): 465-473. |
| 44 | Li L, Liu K, Hu Y, et al. Single mutations convert an outward K+ channel into an inward K+ channel. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(8): 2871-2876. |
| 45 | Gajdanowicz P, Garcia-Mata C, Gonzalez W, et al. Distinct roles of the last transmembrane domain in controlling Arabidopsis K+ channel activity. New Phyologist, 2009, 182(2): 380-391. |
| 46 | Daram P, Urbach S, Gaymard F, et al. Tetramerization of the AKT1 plant potassium channel involves its C-terminal cytoplasmic domain. The EMBO Journal, 1997, 16(12): 3455-3463. |
| 47 | Uozumi N, Nakamura T, Schroeder J I, et al. Determination of transmembrane topology of an inward-rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(17): 9773-9778. |
| 48 | Dreyer I, Blatt M R. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends in Plant Science, 2009, 14(7): 383-390. |
| 49 | Lefoulon C. The bare necessities of plant K+ channel regulation. Plant Physiology, 2021, 187(4): 2092-2109. |
| 50 | Jegla T, Busey G W, Assmann S M. Evolution and structural characteristics of plant voltage-gated K+ channels. The Plant Cell, 2018, 30(12): 2898-2909. |
| 51 | Nieves-Cordones M, Gaillard I. Involvement of the S4-S5 linker and the C-linker domain regions to voltage-gating in plant shaker channels: Comparison with animal HCN and Kv channels. Plant Signaling & Behavior, 2014, 9(10): e972892. |
| 52 | Bassetto C A, Carvalho-de-Souza J L, Bezanilla F. Molecular basis for functional connectivity between the voltage sensor and the selectivity filter gate in shaker K+ channels. eLife, 2021, 10: e63077. |
| 53 | Kalstrup T, Blunck R. S4-S5 linker movement during activation and inactivation in voltage-gated K+ channels. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(29): 6751-6759. |
| 54 | James Z M, Zagotta W N. Structural insights into the mechanisms of CNBD channel function. Journal of General Physiology, 2018, 150(2): 225-244. |
| 55 | Lee S C, Lan W Z, Kim B G, et al. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proceedings of the National Academy of Sciences, 2007, 104(40): 15959-15964. |
| 56 | Dreyer I, Porée F, Schneider A, et al. Assembly of plant shaker-like Kout channels requires two distinct sites of the channel alpha-subunit. Biophysical Journal, 2004, 87(2): 858-872. |
| 57 | Li S, Yang F, Sun D, et al. Cryo-EM structure of the hyperpolarization-activated inwardly rectifying potassium channel KAT1 from Arabidopsis. Cell Research, 2020, 30(11): 1049-1052. |
| 58 | Lin S H, Kuo H F, Canivenc G, et al. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. The Plant Cell, 2008, 20(9): 2514-2528. |
| 59 | Dreyer I, Sussmilch F C, Fukushima K, et al. How to grow a tree: Plant voltage-dependent cation channels in the spotlight of evolution. Trends in Plant Science, 2021, 26(1): 41-52. |
| 60 | Zahoor R, Dong H, Abid M, et al. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environmental and Experimental Botany, 2017, 137(2): 73-83. |
| 61 | Lhamo D, Luan S. Potential networks of nitrogen-phosphorus-potassium channels and transporters in Arabidopsis roots at a single cell resolution. Frontiers in Plant Science, 2021, 12: 689545. |
| 62 | Sharma T, Dreyer I, Riedelsberger J. The role of K+ channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Frontiers in Plant Science, 2013, 4: 224. |
| 63 | Sano T, Becker D, Ivashikina N, et al. Plant cells must pass a K+ threshold to re-enter the cell cycle. The Plant Journal, 2007, 50(3): 401-413. |
| 64 | Eida A A, Alzubaidy H S, de Zelicourt A, et al. Phylogenetically diverse endophytic bacteria from desert plants induce transcriptional changes of tissue-specific ion transporters and salinity stress in Arabidopsis thaliana. Plant Science, 2019, 280(1): 228-240. |
| 65 | White P J. Chapter 2-Ion uptake mechanisms of individual cells and roots: Short-distance transport//Marschner P. Marschner’s mineral nutrition of higher plants (Third Edition). San Diego: Academic Press, 2012: 7-47. |
| 66 | Ahmad I, Maathuis F J. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. Journal of Plant Physiology, 2014, 171(9): 708-714. |
| 67 | Nieves-Cordones M, Lara A, Rόdenas R, et al. Modulation of K+ translocation by AKT1 and AtHAK5 in Arabidopsis plants. Plant Cell and Environment, 2019, 42(8): 2357-2371. |
| 68 | Ahanger M A, Tomar N S, Tittal M, et al. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiology and Molecular Biology of Plants, 2017, 23(4): 731-744. |
| 69 | Demidchik V. Reactive oxygen species, oxidative stress and plant ion channels//Demidchik V, Maathuis F. Ion channels and plant stress responses, signaling and communication in plants. Berlin Heidelberg: Springer, 2010: 207-232. |
| 70 | Demidchik V. Mechanisms and physiological roles of K+ efflux from root cells. Journal of Plant Physiology, 2014, 171(9): 696-707. |
| 71 | Demidchik V, Shabala S N, Coutts K B, et al. Free oxygen radicals regulate plasma membrane Ca2+- and K+- permeable channels in plant root cells. Journal of Cell Science, 2003, 116(1): 81-88. |
| 72 | Demidchik V, Cuin T A, Svistunenko D, et al. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science, 2010, 123(9): 1468-1479. |
| 73 | Garcia-Mata C, Wang J, Gajdanowicz P, et al. A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. Journal of Biological Chemistry, 2010, 285(38): 29286-29294. |
| 74 | Kimura S, Waszczak C, Hunter K, et al. Bound by fate: The role of reactive oxygen species in receptor-like kinase signaling. The Plant Cell, 2017, 29(4): 638-654. |
| 75 | Adem G D, Chen G, Shabala L, et al. GORK channel: A master switch of plant metabolism?Trends in Plant Science, 2020, 25(5): 434-445. |
| 76 | Lacombe B, Pilot G, Gaymard F, et al. pH control of the plant outwardly-rectifying potassium channel SKOR. FEBS Letters, 2000, 466(2): 351-354. |
| 77 | Yu Z P, Duan X B, Luo L, et al. How plant hormones mediate salt stress responses. Trends in Plant Science, 2020, 25(11): 1117-1130. |
| 78 | Roberts S K. Regulation of K+ channels in maize roots by water stress and abscisic acid. Plant Physiology, 1998, 116(1): 145-153. |
| 79 | Wolf T, Heidelmann T, Marten I J P, et al. ABA regulation of K+-permeable channels in maize subsidiary cells. Plant Cell Physiology, 2006, 47(10): 1372-1380. |
| 80 | Ooi A S L, Lemtiri-Chlieh F, Wong A T, et al. Direct modulation of the guard cell outward-rectifying potassium channel (GORK) by abscisic acid. Molecular Plant, 2017, 10(11): 1469-1472. |
| 81 | Rao S, Tian Y, Xia X, et al. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Horticulture Research, 2020, 7(1): 40. |
| 82 | Zhao P S, Yang S Y, Hao D L, et al. Genotypic characteristics and molecular mechanism of potassium nutrition in rice. Soils, 2021, 311(1): 37-46. |
| 赵鹏姝, 杨顺瑛, 郝东利, 等. 水稻钾素营养的基因型特征及分子机制初探. 土壤, 2021, 311(1): 37-46. | |
| 83 | Ma W, Yang G, Xiao Y, et al. ABA-dependent K+ flux is one of the important features of the drought response that distinguishes Catalpa from two different habitats. Plant Signaling & Behavior, 2020, 15(4):1735755. |
| 84 | Pilot G, Gaymard F, Mouline K, et al. Regulated expression of Arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Molecular Biology, 2003, 51(5): 773-787. |
| 85 | Deng Y Q, Bao J, Yuan F, et al. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regulation, 2015, 79(3): 391-399. |
| 86 | Khanna K, Sharma N, Kour S, et al. Hydrogen sulfide: A robust combatant against abiotic stresses in plants. Hydrogen, 2021, 2(3): 319-342. |
| 87 | Janicka M, Reda M, Czyżewska K, et al. Involvement of signalling molecules NO, H2O2 and H2S in modification of plasma membrane proton pump in cucumber roots subjected to salt or low temperature stress. Functional Plant Biology, 2017, 45(4): 428-439. |
| 88 | Zhao N, Zhu H, Zhang H, et al. Hydrogen sulfide mediates K+ and Na+ homeostasis in the roots of salt-resistant and salt-sensitive poplar species subjected to NaCl stress. Frontiers in Plant Science, 2018, 9: 1366. |
| 89 | Li H, Shi J, Wang Z, et al. H2S pretreatment mitigates the alkaline salt stress on Malus hupehensis roots by regulating Na+/K+ homeostasis and oxidative stress. Plant Physiology and Biochemistry, 2020, 156: 233-241. |
| 90 | Wang Z P. Effects of NaHS on root ion flow and related gene expression in M. hupehensis under NaCl stress. Tai’an: Shandong Agricultural University, 2020. |
| 王泽鹏. NaHS对NaCl胁迫下平邑甜茶根系离子流及相关基因表达的影响.泰安: 山东农业大学, 2020. | |
| 91 | Lai D, Mao Y, Zhou H, et al. Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Science, 2014, 225(1): 117-129. |
| 92 | Jiang J L, Tian Y, Li L, et al. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Frontiers in Plant Science, 2019, 10: 678. |
| 93 | Jin Z, Xue S, Luo Y, et al. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiology and Biochemistry, 2013, 62: 41-46. |
| 94 | Schmidt R, Mieulet D, Hubberten H M, et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. The Plant Cell, 2013, 25(6): 2115-2131. |
| 95 | Zhou Y T, Guo Q, Mao P C, et al. Cloning, expression and bioinformatics analysis of EeSKOR gene fragment from Elytrigia elongata. Genomics and Applied Biology, 2020, 39(10): 4686-4694. |
| 周妍彤, 郭强, 毛培春, 等. 长穗偃麦草 EeSKOR 基因片段的克隆、表达及其生物信息学分析.基因组学与应用生物学, 2020, 39(10): 4686-4694. | |
| 96 | Li S J, Wu G Q, Lin L Y. AKT1, HAK5, SKOR, HKT1;5, SOS1 and NHX1 synergistically control Na+ and K+ homeostasis in sugar beet (Beta vulgaris L.) seedlings under saline conditions. Journal of Plant Biochemistry and Biotechnology, 2022, 31(1): 71-84. |
| 97 | Jin Y M, Li Y, Yang H, et al. Identification of shaker gene family in Pyrus betulaefolia and its expression under low potassium and salt stress. Journal of Nanjing Agricultural University, 2021, 196(5): 876-886. |
| 金雨濛, 李岩, 杨晗, 等. 梨Shaker基因家族的鉴定及其响应低钾和盐胁迫的表达分析.南京农业大学学报, 2021, 196(5): 876-886. | |
| 98 | Li Y, Zheng X, Tian Y, et al. Comparative transcriptome analysis of NaCl and KCl stress response in Malus hupehensis Rehd. provide insight into the regulation involved in Na+ and K+ homeostasis. Plant Physiology and Biochemistry, 2021, 164: 101-114. |
| 99 | Hu J, Hu X, Zhang H, et al. Moderate NaCl alleviates osmotic stress in Lycium Ruthenicum. Plant Growth Regulation, 2022, 96: 25-35. |
| 100 | Song X. The key gene excavation and association analysis with salinity tolerance in perennial ryegrass. Lanzhou: Lanzhou University, 2019. |
| 宋鑫. 多年生黑麦草耐盐关键基因的挖掘与关联分析. 兰州: 兰州大学, 2019. | |
| 101 | Zhou J, Nguyen T H, Luu D T, et al. The outward shaker channel OsK5.2 improves plant salt tolerance by contributing to control of both leaf transpiration and K+ secretion into xylem sap. Plant Cell & Environment. 2022, 45(6): 1734-1748. |
| 102 | Maathuis F J. The role of monovalent cation transporters in plant responses to salinity. Journal of Experimental Botany, 2006, 57(5): 1137-1147. |
| 103 | Shabala S, Cuin T A. Potassium transport and plant salt tolerance. Physiology Plant, 2008, 133(4): 651-669. |
| 104 | Khan M I R, Asgher M, Fatma M, et al. Drought stress vis a vis plant functions in the era of climate change. Climate Change and Environmental Sustainability, 2015, 3(1): 13-25. |
| 105 | Huang W G, Jiang W D, Yao Y B, et al. Transcriptome profiling of flax (Linum usttatissimum L.) response to low potassium stress. Acta Agronomica Sinica, 2021, 47(6): 1070-1081. |
| 黄文功, 姜卫东, 姚玉波, 等. 亚麻响应低钾胁迫转录谱分析.作物学报, 2021, 47(6): 1070-1081. | |
| 106 | Jin R, Zhang A, Sun J, et al. Identification of shaker K+ channel family members in sweet potato and functional exploration of IbAKT1. Gene, 2021, 768(5): 145311. |
| 107 | Gao Y L, Sui X Y, Wang B W, et al. Cloning and function analysis of NtSKOR1 in tobacco. Acta Botanica Boreali-Occidentalia Sinica, 2020, 40(12): 2017-2022. |
| 高玉龙, 隋学艺, 王丙武, 等. 烟草NtSKOR1基因的克隆及功能分析.西北植物学报, 2020, 40(12): 2017-2022. | |
| 108 | Armengaud P, Breitling R, Amtmann A. The potassium-dependent transcriptome of Arabidopsis reveals a prominent role of jasmonic acid in nutrient signaling. Plant physiology, 2004, 136(1): 2556-2576. |
| 109 | Rόdenas R, García-Legaz M F, Lόpez-Gόmez E, et al. NO3 -, PO4 3- and SO4 2- deprivation reduced LKT1-mediated low-affinity K+ uptake and SKOR-mediated K+ translocation in tomato and Arabidopsis plants. Physiology Plantarum, 2017, 160(4): 410-424. |
| 110 | Dijkshoorn W, Lathwell D J, De Wit C T. Temporal changes in carboxylate content of ryegrass with stepwise change in nutrition. Plant and Soil, 1968, 29(3): 369-390. |
| 111 | Drechsler N, Zheng Y, Bohner A, et al. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiology, 2015, 169(4): 2832-2847. |
| 112 | Fang X Z, Liu X X, Zhu Y X, et al. The K+ and NO3 - interaction mediated by NITRATE TRANSPORTER1.1 ensures better plant growth under K+-limiting conditions. Plant Physiology, 2020, 184(4): 1900-1916. |
| [1] | 李佳林, 姜升林, 李云霞, 全晓艳, 王文波, 单秋丽, 解纯娟, 尹宁, 秦余香, 张立华, 李洪梅, 何文兴. 禾本科草本植物根状茎发育调控机理研究进展[J]. 草业学报, 2022, 31(8): 211-220. |
| [2] | 童长春, 刘晓静, 吴勇, 赵雅姣, 王静. 内源异黄酮对紫花苜蓿结瘤固氮及氮效率的调控研究[J]. 草业学报, 2022, 31(3): 124-135. |
| [3] | 姜渊博, 康燕霞, 齐广平, 银敏华, 马彦麟, 汪精海, 贾琼, 康瑶, 张宏斌, 唐仲霞, 汪爱霞. 基于产量与品质的无芒雀麦灌溉制度研究[J]. 草业学报, 2022, 31(11): 158-171. |
| [4] | 陆姣云,段兵红,杨梅,杨晗,杨惠敏. 植物叶片氮磷养分重吸收规律及其调控机制研究进展[J]. 草业学报, 2018, 27(4): 178-188. |
| [5] | 杨梅, 王亚亚, 陆姣云, 刘敏国, 段兵红, 杨惠敏. 典型果园生草模式及果草系统资源调控研究进展[J]. 草业学报, 2017, 26(9): 189-199. |
| [6] | 张永超, 袁晓波, 牛得草, 吴淑娟, 张典业, 宗文杰, 傅华. 玛曲高寒草甸高原鼠兔种群数量对植被调控措施的响应[J]. 草业学报, 2016, 25(2): 87-94. |
| [7] | 董臣飞, 顾洪如, 丁成龙, 许能祥, 张文洁. 水稻生育后期外源赤霉素调控稻草饲用品质的机理研究[J]. 草业学报, 2016, 25(11): 94-102. |
| [8] | 刘强, 张锁科, 孙万斌, 俞玲, 马晖玲. 不同营养调控对草地早熟禾生长和内源激素含量影响研究[J]. 草业学报, 2015, 24(2): 31-40. |
| [9] | 刘芳,王家艳,周蕴薇. 球根植物休眠调控的分子机制研究进展[J]. 草业学报, 2013, 22(6): 295-304. |
| [10] | 蒋乔峰,陈静波,宗俊勤,李珊,褚晓晴,郭海林,刘建秀. 盐胁迫下磷素对沟叶结缕草生长及Na+和K+含量的影响[J]. 草业学报, 2013, 22(3): 162-. |
| [11] | 刘志鹏,张吉宇,王彦荣. 紫花苜蓿配子体发育遗传调控的研究进展[J]. 草业学报, 2011, 20(4): 270-278. |
| [12] | 张天瑞,皇甫超河,杨殿林,白小明. 外来植物黄顶菊的入侵机制及生态调控技术研究进展[J]. 草业学报, 2011, 20(3): 268-278. |
| [13] | 张晓艳,刘锋,王风云,董树亭. 墨西哥玉米留茬中糖组分含量对氮素的响应[J]. 草业学报, 2009, 18(1): 184-187. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||