

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2021, Vol. 30 ›› Issue (11): 157-169.DOI: 10.11686/cyxb2020426
• 研究论文 • 上一篇
收稿日期:2020-09-21
修回日期:2020-12-07
出版日期:2021-10-19
发布日期:2021-10-19
通讯作者:
安渊
作者简介:Corresponding author. E-mail: anyuan@sjtu.edu.cn基金资助:
Ru-yue WANG( ), Wu-wu WEN, En-hua ZHAO, Peng ZHOU, Yuan AN(
), Wu-wu WEN, En-hua ZHAO, Peng ZHOU, Yuan AN( )
)
Received:2020-09-21
Revised:2020-12-07
Online:2021-10-19
Published:2021-10-19
Contact:
Yuan AN
摘要:
本研究从紫花苜蓿cDNA中克隆到一个MsWRKY11基因,进化分析表明MsWRKY11与蒺藜苜蓿WRKY11亲缘关系最近。MsWRKY11受NaCl、水杨酸(SA)和茉莉酸(JA)的诱导表达。将PHB-MsWRKY11-Flag表达载体转入紫花苜蓿获得超表达紫花苜蓿。250 mmol·L-1 NaCl处理下,转基因株系的株高和地上生物量,以及过氧化氢酶(CAT)、过氧化物酶(POD)活性和K+/Na+均明显高于野生型,而Na+含量、电导率、丙二醛(MDA)含量和超氧阴离子含量均显著低于野生型。进一步分析发现盐胁迫下耐盐性相关的关键基因MsNHX1、MsSOS3、MsAPX、MsGRX、MsNAC和MsP5CS的表达量受到强烈诱导,转基因紫花苜蓿叶片中6个基因的表达量高于野生型。上述结果表明,MsWRKY11通过调节细胞K+/Na+内稳态和保护过氧化物酶活性,从而降低Na+对细胞膜结构的破坏,提高紫花苜蓿的耐盐能力。
王如月, 文武武, 赵恩华, 周鹏, 安渊. 紫花苜蓿MsWRKY11基因的克隆及其耐盐功能分析[J]. 草业学报, 2021, 30(11): 157-169.
Ru-yue WANG, Wu-wu WEN, En-hua ZHAO, Peng ZHOU, Yuan AN. Cloning and salt-tolerance analysis of MsWRKY11 in alfalfa[J]. Acta Prataculturae Sinica, 2021, 30(11): 157-169.
耐盐性相关基因 Salt-related gene | 正向引物(5′-3′) Forward primer (5′-3′) | 反向引物(3′-5′) Reverse primer (3′-5′) |
|---|---|---|
| EF-α | GCACCAGTGCTCGATTGC | TCGCCTGTCAATCTTGGTAACAA |
| MsWRKY11 | CTTATAGCATCTCTACCACTAC | CTTGCCAGAGGAAATGATAG |
| MsNHX1 | GCCTTCGTGCTTTACTATCAAC | GATTACCATTGCGTTCACTTGG |
| MsSOS3 | GTTCTTGCTTCTGAAACACC | CCAAGAGATCGAACAAATTC |
| MsAPX | TCGGAACCATCAAGCACCAAGC | CAACAGCAACAACACCAGCCAAC |
| MsGRX | GCTGCCCAACTGTCCACCAAC | TGACTTGCCATGACTCTTTCC |
| MsNAC | GAAAGACTGGGATAGCGAAGAG | CTAGGTAGAATGAGAGCTGGTG |
| MsP5CS | CCTCGGTCGACAAAGGCTTA | CCCCCTCTTCCAACCCTAGA |
表1 qRT-PCR 引物序列
Table 1 qRT-PCR primer sequences
耐盐性相关基因 Salt-related gene | 正向引物(5′-3′) Forward primer (5′-3′) | 反向引物(3′-5′) Reverse primer (3′-5′) |
|---|---|---|
| EF-α | GCACCAGTGCTCGATTGC | TCGCCTGTCAATCTTGGTAACAA |
| MsWRKY11 | CTTATAGCATCTCTACCACTAC | CTTGCCAGAGGAAATGATAG |
| MsNHX1 | GCCTTCGTGCTTTACTATCAAC | GATTACCATTGCGTTCACTTGG |
| MsSOS3 | GTTCTTGCTTCTGAAACACC | CCAAGAGATCGAACAAATTC |
| MsAPX | TCGGAACCATCAAGCACCAAGC | CAACAGCAACAACACCAGCCAAC |
| MsGRX | GCTGCCCAACTGTCCACCAAC | TGACTTGCCATGACTCTTTCC |
| MsNAC | GAAAGACTGGGATAGCGAAGAG | CTAGGTAGAATGAGAGCTGGTG |
| MsP5CS | CCTCGGTCGACAAAGGCTTA | CCCCCTCTTCCAACCCTAGA |

图1 MsWRKY11的克隆及序列比对A: MsWRKY11基因cDNA全长扩增条带,1~6表示不同引物或不同Tm值扩增条带;B:MsWRKY11(Query)与MtWRKY11(Sbjct)序列比对结果。A: Banding of MsWRKY11 gene, 1-6 indicated different primers or Tm value amplification bands; B: Sequence alignment results of MsWRKY11 (Query) and MtWRKY11 (Sbjct).
Fig.1 The cloning and sequence alignment of MsWRKY11
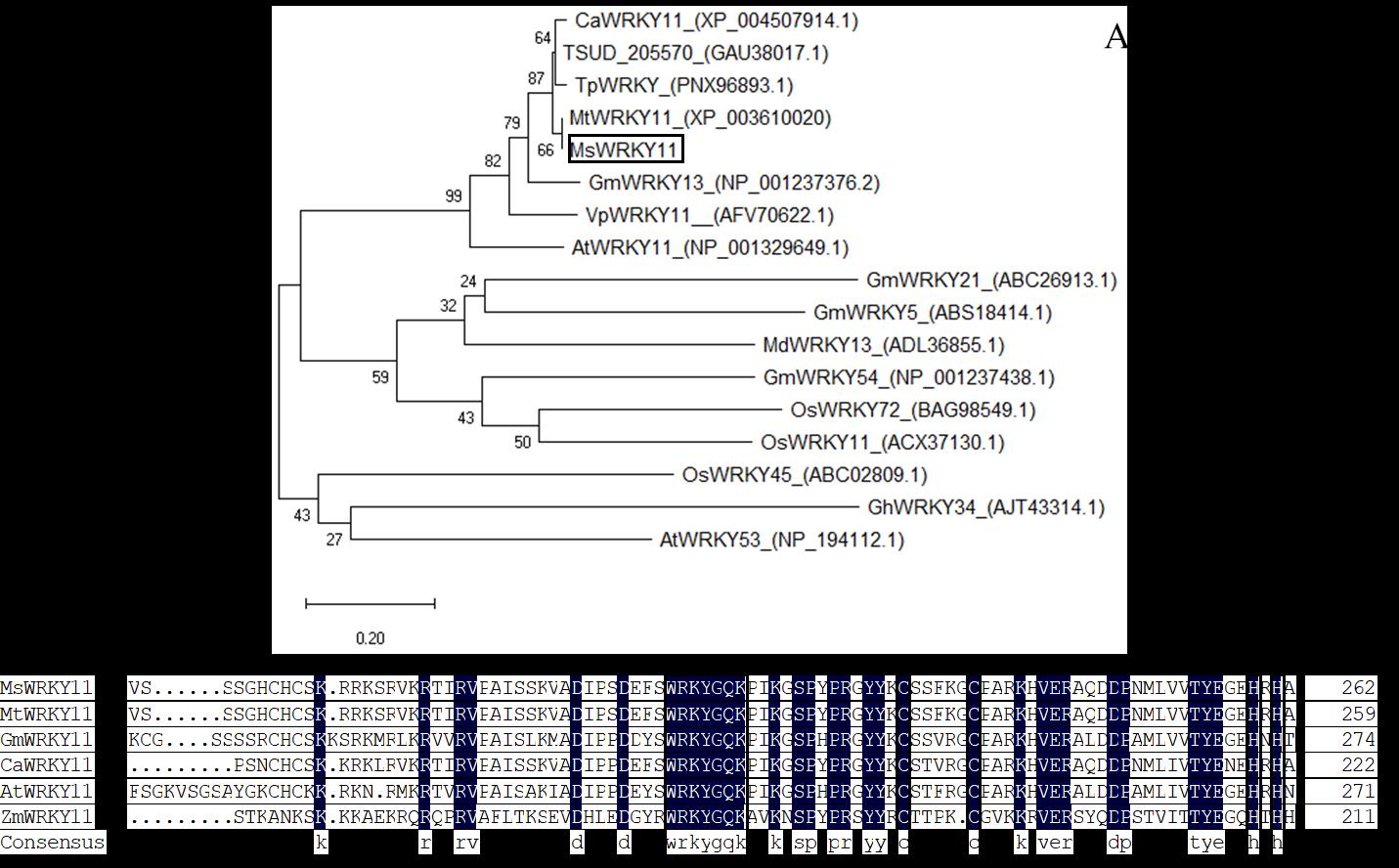
图2 MsWRKY11系统进化树分析与同源序列比对A: MsWRKY11系统进化树分析, 数字代表可信度; B: MsWRKY11同源序列比对,方框中为WRKYGQK和C2H2结构域,数字代表碱基在MsWRKY11中的位置。A: Phylogenetic tree analysis of MsWRKY11, the number represents credibility; B: Homologous sequence alignment of MsWRKY11. In the box are WRKYGQK and C2H2 domains. The number represents the position of the base in MsWRKY11.
Fig.2 Analysis of MsWRKY11 evolutionary tree and sequence alignment

图3 MsWRKY11蛋白结构预测A: MsWRKY11蛋白二级结构预测;B: MsWRKY11蛋白三级结构预测。A: The secondary structure prediction of MsWRKY11 protein; B: The tertiary structure prediction of MsWRKY11 protein.
Fig.3 MsWRKY11 protein structure prediction
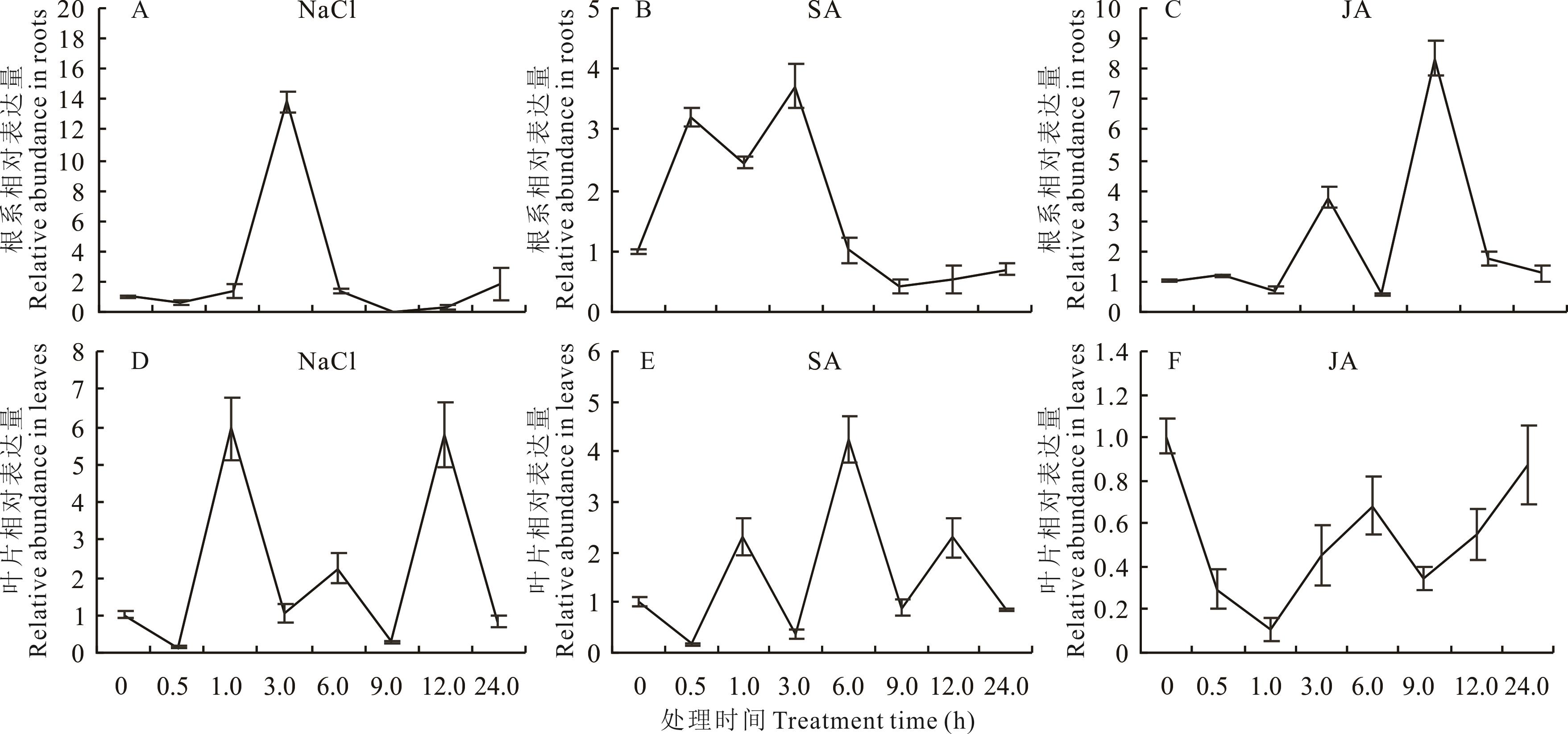
图4 NaCl、SA和JA处理下MsWRKY11在根和叶片中的表达模式A~C:分别为200 mmol·L-1 NaCl、100 mmol·L-1 SA、100 mmol·L-1 JA处理0~24 h,紫花苜蓿根系MsWRKY11的表达量; D~F: 分别为200 mmol·L-1 NaCl、100 mmol·L-1 SA、100 mmol·L-1 JA处理0~24 h,紫花苜蓿叶片MsWRKY11的表达量。A-C: The expression of MsWRKY11 in alfalfa roots treated with 200 mmol·L-1 NaCl, 100 mmol·L-1 SA and 100 mmol·L-1 JA for 0-24 h; D-F: The expression of MsWRKY11 in alfalfa leaves treated with 200 mmol·L-1 NaCl, 100 mmol·L-1 SA and 100 mmol·L-1 JA for 0-24 h.
Fig.4 The expression pattern of MsWRKY11 in leaves and roots under NaCl, SA and JA treatment
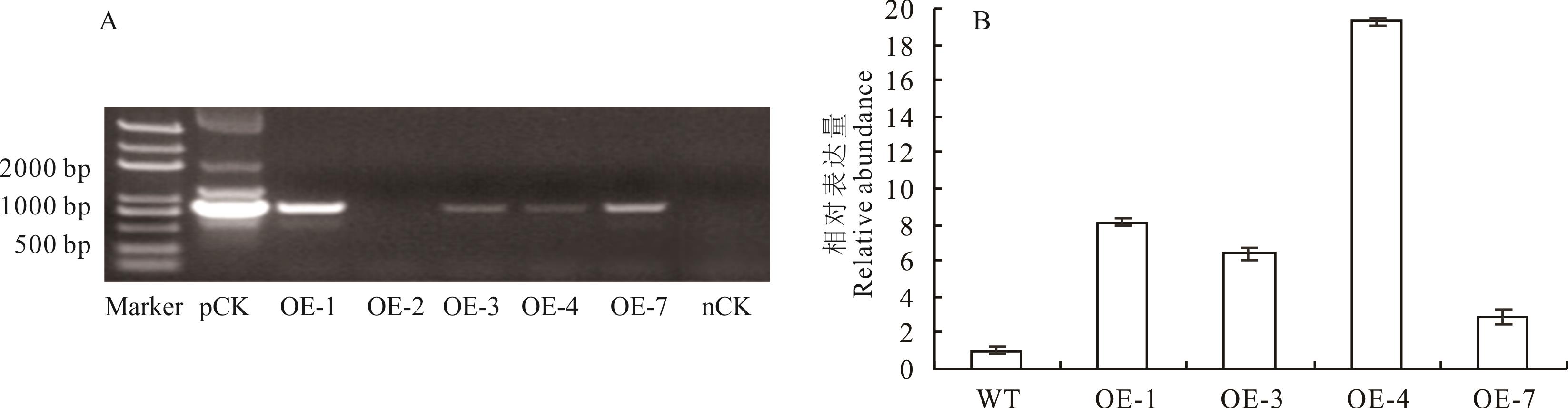
图5 转MsWRKY11紫花苜蓿植株鉴定A: 转基因株系DNA扩增条带,pCK为阳性对照,OE-1、2、3、4、7为MsWRKY11转基因株系,nCK为阴性对照;B: MsWRKY11转基因株系表达量,WT为野生型,OE-1/3/4/7为MsWRKY11转基因株系。A: DNA amplification bands of transgenic lines. pCK was positive control; OE-1, 2, 3, 4, 7 were MsWRKY11 transgenic lines; nCK was negative control; B: Expression level of MsWRKY11 transgenic lines; WT was wild type; OE-1/3/4/7 were MsWRKY11 transgenic lines.
Fig.5 Identification of transgenic alfalfa

图6 盐胁迫下MsWRKY11转基因株系的表型变化A:野生型(WT)和MsWRKY11超表达紫花苜蓿株系(OE-1、OE-3、OE-4、OE-7)在正常条件下的生长表型;B:WT和转基因株系在250 mmol·L-1 NaCl处理下的生长表型;C、D: WT和转基因株系在对照和250 mmol·L-1 NaCl处理下的地上生物量和株高。A: Growth phenotypes of wild type (WT) and MsWRKY11 over expressed alfalfa lines (OE-1, OE-3, OE-4, OE-7) under normal conditions; B: Growth phenotypes of WT and transgenic lines treated with 250 mmol·L-1 NaCl; C, D: Aboveground biomass and plant height of WT and transgenic lines treated with 250 mmol·L-1 NaCl and control. 同一处理不同字母表示差异显著(P<0.05),下同。The different letters under the same treatment indicate significant difference at the 0.05 level, the same below.
Fig.6 Phenotype analysis of MsWRKY11 transgenic lines under salt stress
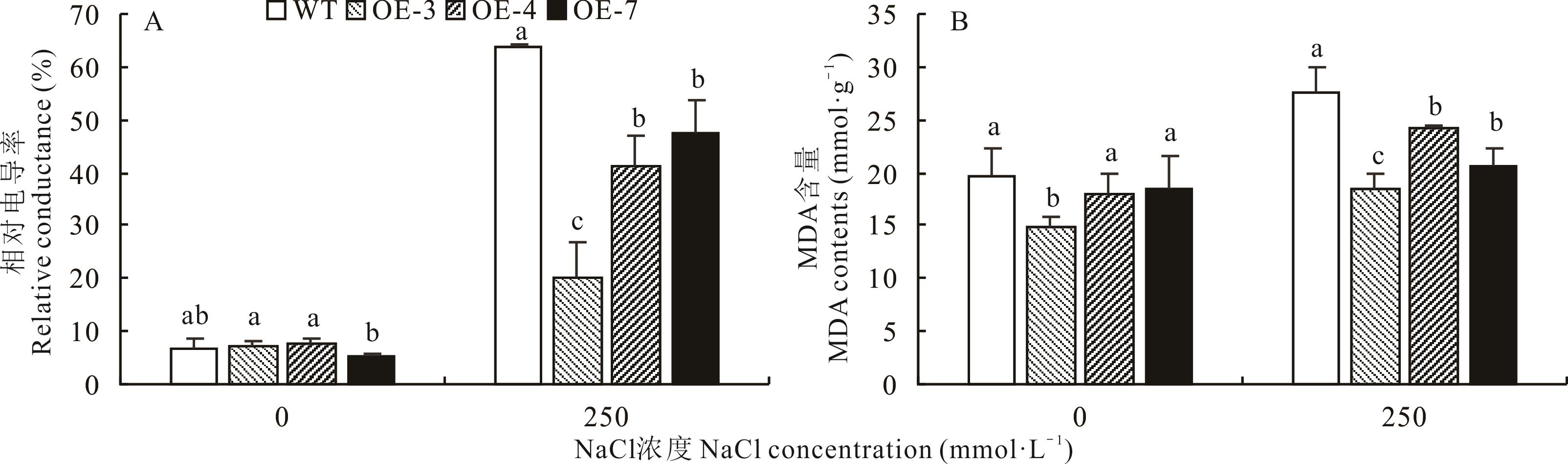
图7 盐胁迫对过表达MsWRKY11紫花苜蓿细胞膜脂过氧化的影响A、B: WT和转基因株系在对照和250 mmol·L-1 NaCl处理下叶片的相对电导率和MDA含量。A, B: The relative conductivity and MDA content of leaves of WT and transgenic lines treated with 250 mmol·L-1 NaCl and control.
Fig.7 Effect of overexpression of MsWRKY11 on membrane lipid peroxidation of alfalfa under salt stress

图 8 过表达MsWRKY11对盐胁迫下紫花苜蓿抗氧化胁迫能力的影响A~D: 分别为WT和转基因株系在对照和250 mmol·L-1 NaCl处理下叶片NBT染色情况、超氧阴离子含量、POD活性和CAT活性。A-D: NBT staining, superoxide anion content, POD activity and CAT activity in leaves of WT and transgenic lines under control and 250 mmol·L-1 NaCl treatment, respectively.
Fig.8 Effects of overexpression of MsWRKY11 on alfalfa antioxidant ability under salt stress

图 9 盐胁迫下转基因紫花苜蓿幼苗的K+、Na+含量和K+/Na+A和B: 250 mmol·L-1 NaCl处理下根系和叶片中Na+含量;C和D: 250 mmol·L-1 NaCl处理下根系和叶片中K+含量;E和F: 250 mmol·L-1 NaCl处理下根系和叶片中K+/Na+。A and B: Na+ content in roots and leaves under 250 mmol·L-1 NaCl treatment; C and D: K+ content in roots and leaves under 250 mmol·L-1 NaCl treatment; E and F: K+/Na+ in roots and leaves under 250 mmol·L-1 NaCl treatment.
Fig.9 Content of K+, Na+ and ratio of K+/Na+ in alfalfa seedlings under salt stress
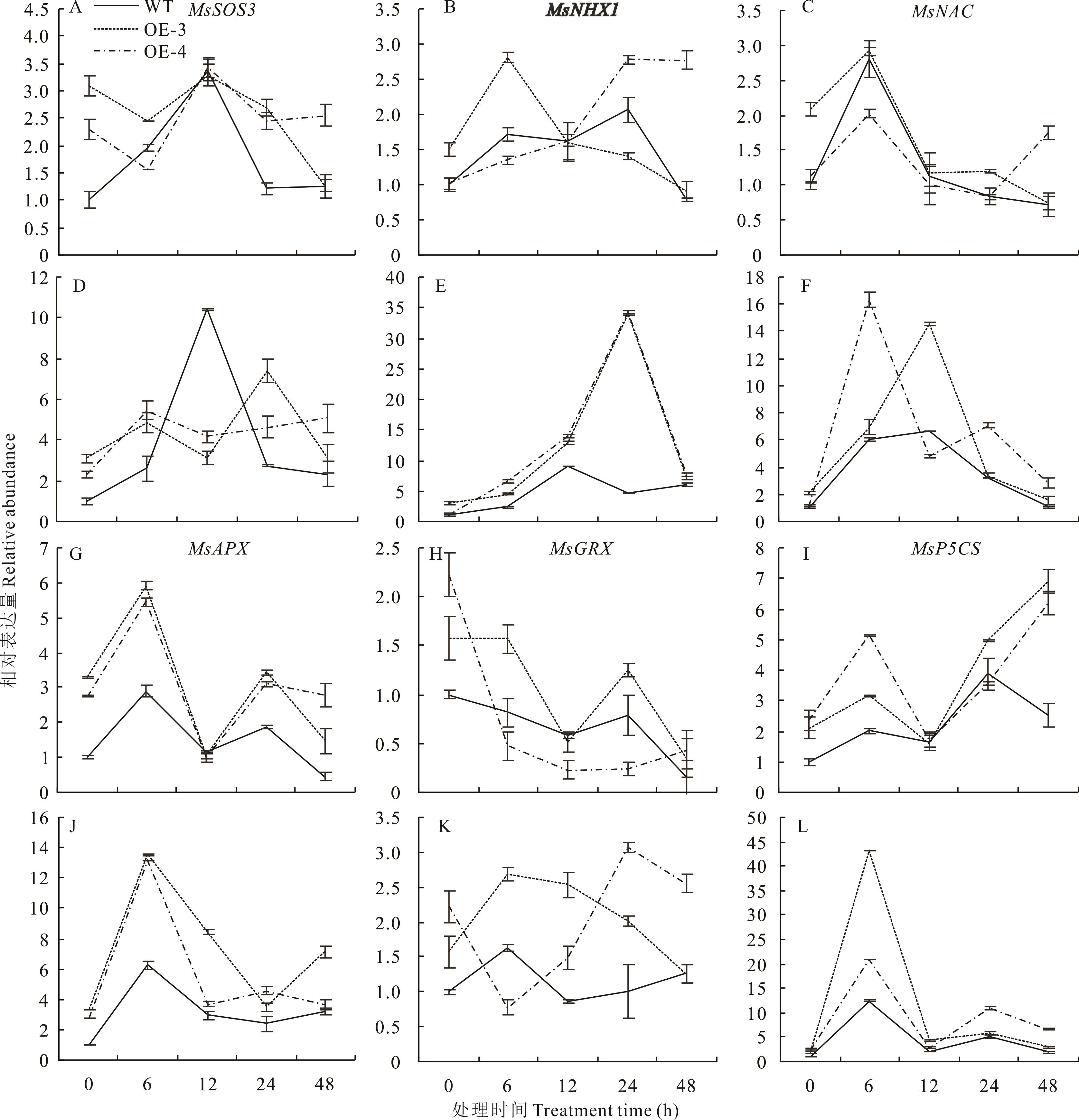
图 10 盐胁迫下转基因紫花苜蓿耐盐相关基因的表达变化A~C、G~I:分别为对照处理0,6,12,24,48 h野生型(WT)和转基因株系(OE-3、OE-4)叶片中MsSOS3、MsNHX1、MsNAC、MsAPX、MsGRX和MsP5CS的相对表达量;D~F、J~L:分别为200 mmol·L-1 NaCl处理0,6,12,24,48 h野生型(WT)和转基因株系(OE-3、OE-4)叶片中MsSOS3、MsNHX1、MsNAC、MsAPX、MsGRX和MsP5CS的相对表达量。A-C and G-I: The relative abundance of MsSOS3, MsNHx1, MsNAC, MsAPX, MsGRX and MsP5CS in the leaves of wild-type (WT) and transgenic lines (OE-3 and OE-4) at 0, 6, 12, 24 and 48 h, respectively. D-F and J-L: The relative abundance of MsSOS3, MsNHx1, MsNAC, MsAPX, MsGRX and MsP5CS in leaves of wild-type (WT) and transgenic lines (OE-3 and OE-4) treated with 200 mmol·L-1 NaCl at 0, 6, 12, 24 and 48 h, respectively.
Fig.10 Expression patterns of key genes related to salt stress in transgenic alfalfa under salt stress
| 1 | Meng F H, Wang C, Xu S J. Advances in research on effects of salt stress on plant and the mechanism of plant salt tolerance. Journal of Inner Mongolia University for Nationalities (Natural Sciences), 2014, 29(3): 315-318. |
| 孟繁昊, 王聪, 徐寿军. 盐胁迫对植物的影响及植物耐盐机理研究进展. 内蒙古民族大学学报(自然科学版), 2014, 29(3): 315-318. | |
| 2 | Yang Z R, Wang X C, Li X M, et al. Advance on the study of transcription factors in higher plants. Hereditas (Beijing), 2004, 26(3): 403-408. |
| 杨致荣, 王兴春, 李西明, 等. 高等植物转录因子的研究进展. 遗传, 2004, 26(3): 403-408. | |
| 3 | Wu L T, Zhong G M, Wang J M, et al. Arabidopsis WRKY28 transcription factor is required for resistance to necrotrophic pathogen, Botrytis cinerea. African Journal of Microbiology Research, 2011, 5(30): 5481-5488. |
| 4 | Rushton D L, Tripathi P, Rabara R C, et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnology Journal, 2012, 10(1): 2-11. |
| 5 | Zou C, Jiang W, Yu D. Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. Journal of Experimental Botany, 2010, 61(14): 3901-3914. |
| 6 | Eulgem T, Rushton P, Robatzek S, et al. The WRKY superfamily of plant transcription factors. Trend in Plant Science, 2000, 5(5): 199-206. |
| 7 | Ülker B, Somssich I E. WRKY transcription factors: From DNA binding towards biological function. Current Opinion in Plant Biology, 2004, 7(5): 491-498. |
| 8 | Zhou L, Wang N N, Gong S Y, et al. Overexpression of a cotton (Gossypium hirsutum) WRKY gene, GhWRKY34, in Arabidopsis enhances salt-tolerance of the transgenic plants. Plant Physiology and Biochemistry, 2015, 96: 311-320. |
| 9 | Yu Y C, Wang N, Hu R B, et al. Genome-wide identification of soybean WRKY transcription factors in response to salt stress. Springerplus, 2016, 5(1): 920-926. |
| 10 | Zhou Q Y, Tian A G, Zou H F, et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnology Journal, 2008, 6(5): 486-503. |
| 11 | Zhang H Y. A study on the characters of content of inorganic ions in salt-stressed Suaeda salsa.Acta Botanica Boreali-Occidentalia Sinica, 2002, 22(1): 129-135. |
| 张海燕. 盐胁迫下盐地碱蓬体内无机离子含量分布特点的研究. 西北植物学报, 2002, 22(1): 129-135. | |
| 12 | Zhen X, Zhang Z L, Hanzlik S, et al. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Molecular Biology, 2007, 64(3): 293-303. |
| 13 | Seki M, Narusaka M, Kamiya A, et al. Functional annotation of a full-length Arabidopsis cDNA collection. Science, 2002, 296(5565): 141-145. |
| 14 | Song Y. The function exploration of four plant WRKY transcription factors. Xishuangbanna: Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, 2008. |
| 宋钰. 四个植物WRKY转录调控因子功能初探.西双版纳: 中国科学院西双版纳热带植物园, 2008. | |
| 15 | Qiu Y P, Yu D Q. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environmental and Experimental Botany, 2009, 65(1): 35-47. |
| 16 | Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany, 2003, 91(5): 503-527. |
| 17 | Yang Q C, Wu M S, Wang P Q, et al. Cloning and expression analysis of a vacuolar Na+/H+ antiporter gene from alfalfa. DNA Sequence, 2005, 16(5): 352-357. |
| 18 | Li K, Zhou Z Y, Li S J, et al. Growth, osmotic adjustment and antioxidant capacity responses of Schizonepeta tenuifolia to drought stress. Acta Prataculturae Sinica, 2020, 29(5): 150-158. |
| 李柯, 周庄煜, 李四菊,等. 荆芥的生长, 渗透调节和抗氧化能力对干旱胁迫的响应. 草业学报, 2020, 29(5): 150-158. | |
| 19 | Li W F, Wang D L, Jin T C, et al. The vacuolar Na+/H+ antiporter gene SsNHX1 from the halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Molecular Biology Reporter, 2011, 29(2): 278-290. |
| 20 | Xiao Z H, Li X H, Pan G, et al. Effects of manganese stress on seed germination, and seedling physiological and biochemical characteristics of Cleome viscosa. Acta Prataculturae Sinica, 2019, 28(12): 75-84. |
| 肖泽华, 李欣航, 潘高,等. 锰胁迫对黄花草种子萌发及幼苗生理生化特征的影响. 草业学报, 2019, 28(12): 75-84. | |
| 21 | Zhao X, Yang X, Pei S, et al. The miscanthus NAC transcription factor MlNAC9 enhances abiotic stress tolerance in transgenic Arabidopsis. Gene, 2016(1): 158-169. |
| 22 | Cao S J. Cloning and salt-resistant function analysis in tobacco of LpNAC6 and LpNAC20 genes from Lilium pumilum.Harbin: Northeast Forestry University, 2019. |
| 曹尚杰. 细叶百合LpNAC6和LpNAC20基因的克隆及其在烟草中的抗盐功能分析.哈尔滨: 东北林业大学, 2019. | |
| 23 | Chinnusamy V, Jagendorf A, Zhu J K. Understanding and improving salt tolerance in plants. Crop Science, 2005, 45(2): 437-448. |
| 24 | Guo Y S, Zhang J H, Zhang J S, et al. Cloning and expression pattern analysis a novel NbGRX1 gene from Nicotiana benthamiana. Journal of Northeast Agricultural University, 2011, 42(10): 47-52. |
| 郭玉双, 张建华, 张吉顺, 等. 烟草谷氧还蛋白基因NbGRX1的克隆与表达特性分析. 东北农业大学学报, 2011, 42(10): 47-52. | |
| 25 | Ma J, Zheng G. Identification and preliminary analysis of salt stress-responsive genes in leaves of southern type alfalfa (Medicago sativa ‘Millennium’). Journal of Agricultural Biotechnology, 2015, 23(12): 1531-1541. |
| 马进, 郑钢. 南方型紫花苜蓿叶片盐胁迫应答基因鉴定与分析. 农业生物技术学报, 2015, 23(12): 1531-1541. | |
| 26 | Li Y J, Hai R L, Du X H, et al. Over-expression of a populus peroxisomal ascorbate peroxidase (PpAPX) gene in tobacco plants enhances stress tolerance. Plant Breeding, 2010, 128(4): 404-410. |
| 27 | Guo Y S, Huang C J, Xie Y, et al. A tomato glutaredoxin gene SlGRX1 regulates plant responses to oxidative, drought and salt stresses. Planta, 2010, 232(6): 1499-1509. |
| 28 | Qiu Q S, Guo Y, Dietrich M A, et al. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(12): 8436-8441. |
| 29 | Apse M P, Aharon G S, Snedden W A, et al. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science, 1999, 285(5431): 1256-1258. |
| 30 | Shi H Z, Lee B H, Wu S J, et al. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnology, 2003, 21(1): 81-85. |
| 31 | Liu M, Wang T Z, Zhang W H. Sodium extrusion associated with enhanced expression of SOS1 underlies different salt tolerance between Medicago falcata and Medicago truncatula seedlings. Environmental & Experimental Botany, 2015, 110: 46-55. |
| 32 | Wu Y Y, Chen Q J, Chen M, et al. Salt-tolerant transgenic perennial ryegrass (Lolium perenne L.) obtained by Agrobacterium tumefaciens-mediated transformation of the vacuolar Na+/H+ antiporter gene. Plant Science, 2005, 169(1): 65-73. |
| 33 | Atsunori F, Kazuhiro C, Miki M, et al. Effect of salt and osmotic stresses on the expression of genes for the vacuolar H+-pyrophosphatase, H+-ATPase subunit A, and Na+/H+ antiporter from barley. Journal of Experimental Botany, 2004, 55(397): 585-594. |
| 34 | Wu G Q, Xi J J, Qian W, et al. The ZxNHX gene encoding tonoplast Na+/H+ antiporter from the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. Journal of Plant Physiology, 2011, 168(8): 758-767. |
| 35 | Jeong J S, Kim Y S, Baek K H, et al. Root-specific expression of osnac10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiology, 2010, 153(1): 185-197. |
| 36 | Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiology, 2009, 149(1): 88-95. |
| 37 | Li L, Chen X, Wang F, et al. Piriformospora indica confers salt tolerance in Medicago sativa by stimulating antioxidant enzymes activities and the expression of P5CS genes. Journal of Hebei University of Technology, 2016(4): 29-36. |
| 李亮, 陈希, 王奋, 等. 印度梨形孢通过激活抗氧化物酶活性及诱导P5CS基因表达提高紫花苜蓿耐盐性. 河北工业大学学报, 2016(4): 29-36. | |
| 38 | Ginzberg I, Stein H, Kapulnik Y, et al. Isolation and characterization of two different cDNAs of delta1-pyrroline-5-carboxylate synthase in alfalfa, transcriptionally induced upon salt stress. Plant Molecular Biology, 1998, 38(5): 755-764. |
| [1] | 赵颖, 辛夏青, 魏小红. 一氧化氮对干旱胁迫下紫花苜蓿氮代谢的影响[J]. 草业学报, 2021, 30(9): 86-96. |
| [2] | 汪雪, 刘晓静, 赵雅姣, 王静. 根系分隔方式下紫花苜蓿/燕麦间作氮素利用及种间互馈特征研究[J]. 草业学报, 2021, 30(8): 73-85. |
| [3] | 古丽娜扎尔·艾力null, 陶海宁, 王自奎, 沈禹颖. 基于APSIM模型的黄土旱塬区苜蓿——小麦轮作系统深层土壤水分及水分利用效率研究[J]. 草业学报, 2021, 30(7): 22-33. |
| [4] | 周倩倩, 张亚见, 张静, 殷涂童, 盛下放, 何琳燕. 产硫化氢细菌的筛选及阻控苜蓿吸收铅和改良土壤的作用[J]. 草业学报, 2021, 30(7): 44-52. |
| [5] | 臧真凤, 白婕, 刘丛, 昝看卓, 龙明秀, 何树斌. 紫花苜蓿形态和生理指标响应干旱胁迫的品种特异性[J]. 草业学报, 2021, 30(6): 73-81. |
| [6] | 谢展, 穆麟, 张志飞, 陈桂华, 刘洋, 高帅, 魏仲珊. 乳酸菌或有机酸盐与尿素复配添加对紫花苜蓿混合青贮的影响[J]. 草业学报, 2021, 30(5): 165-173. |
| [7] | 王吉祥, 宫焕宇, 屠祥建, 郭侲洐, 赵嘉楠, 沈健, 栗振义, 孙娟. 耐亚磷酸盐紫花苜蓿品种筛选及评价指标的鉴定[J]. 草业学报, 2021, 30(5): 186-199. |
| [8] | 张小芳, 魏小红, 刘放, 朱雪妹. PEG胁迫下紫花苜蓿幼苗内源激素对NO的响应[J]. 草业学报, 2021, 30(4): 160-169. |
| [9] | 候怡谣, 李霄, 龙瑞才, 杨青川, 康俊梅, 郭长虹. 过量表达紫花苜蓿MsHB7基因对拟南芥耐旱性的影响[J]. 草业学报, 2021, 30(4): 170-179. |
| [10] | 马欣, 罗珠珠, 张耀全, 刘家鹤, 牛伊宁, 蔡立群. 黄土高原雨养区不同种植年限紫花苜蓿土壤细菌群落特征与生态功能预测[J]. 草业学报, 2021, 30(3): 54-67. |
| [11] | 沙栢平, 谢应忠, 高雪芹, 蔡伟, 伏兵哲. 地下滴灌水肥耦合对紫花苜蓿草产量及品质的影响[J]. 草业学报, 2021, 30(2): 102-114. |
| [12] | 周晶, 陈思齐, 史文娇, 阳伏林, 林辉, 林占熺. 巨菌草幼叶及根转录组功能基因测序及分析[J]. 草业学报, 2021, 30(2): 143-155. |
| [13] | 马倩, 闫启, 张正社, 吴凡, 张吉宇. 紫花苜蓿CCoAOMT基因家族的鉴定、进化及表达分析[J]. 草业学报, 2021, 30(11): 144-156. |
| [14] | 王如月, 袁世力, 文武武, 周鹏, 安渊. 磷对铝胁迫紫花苜蓿幼苗根系生长和生理特征的影响[J]. 草业学报, 2021, 30(10): 53-62. |
| [15] | 李振松, 万里强, 李硕, 李向林. 苜蓿根系构型及生理特性对干旱复水的响应[J]. 草业学报, 2021, 30(1): 189-196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||