

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2022, Vol. 31 ›› Issue (7): 122-132.DOI: 10.11686/cyxb2021178
• 研究论文 • 上一篇
曾令霜1( ), 李培英1,2(
), 李培英1,2( ), 孙宗玖1,2(
), 孙宗玖1,2( ), 孙晓梵1
), 孙晓梵1
收稿日期:2021-05-07
修回日期:2021-10-08
出版日期:2022-07-20
发布日期:2022-06-01
通讯作者:
李培英,孙宗玖
作者简介:nmszj@21cn.com基金资助:
Ling-shuang ZENG1( ), Pei-ying LI1,2(
), Pei-ying LI1,2( ), Zong-jiu SUN1,2(
), Zong-jiu SUN1,2( ), Xiao-fan SUN1
), Xiao-fan SUN1
Received:2021-05-07
Revised:2021-10-08
Online:2022-07-20
Published:2022-06-01
Contact:
Pei-ying LI,Zong-jiu SUN
摘要:
新疆狗牙根具发达的根状茎和细长的匍匐茎,在耐旱性方面表现出广泛的遗传变异特性,对抗旱型C138和不抗旱型C32狗牙根两个基因型进行自然干旱7 d后复水3 d处理,明确2个基因型在干旱胁迫及复水下抗氧化酶系统生理响应及基因表达水平上的差异。结果表明:干旱胁迫降低了狗牙根草坪表观质量、叶片相对含水量和叶绿素含量,电导率及丙二醛(malondialdehyde, MDA)含量升高,且C32较C138下降或升高更为明显。随干旱时间延长,狗牙根体内超氧阴离子自由基(O2·-)、过氧化氢(H2O2)、MDA含量均显著上升,且C32较C138升高更显著,而超氧化物歧化酶(superoxide dismutase, SOD)、过氧化物酶(peroxidase, POD)和过氧化氢酶(catalase, CAT)活性呈先升后降趋势,抗坏血酸过氧化物酶(ascorbate peroxidase, APX)活性呈增加趋势。抗旱型C138对比不抗旱型C32表现为SOD、POD、APX、DHAR和GPX等抗氧化基因高水平的表达,而不抗旱型C32较C138表现出高的脂质过氧化和低的抗氧化酶活性及基因表达水平;在复水过程中,POD活性在提高抗氧化能力及清除活性氧(reactive oxygen species, ROS)过程中起着至关重要的作用。干旱胁迫诱导抗氧化酶及其相关基因上调表达,增强了细胞内的抗氧化防御能力,有助于狗牙根维持细胞膜稳定性,延缓叶片的脱水,从而提高了狗牙根的抗旱能力。
曾令霜, 李培英, 孙宗玖, 孙晓梵. 两类新疆狗牙根抗旱基因型抗氧化酶保护系统及其基因表达差异分析[J]. 草业学报, 2022, 31(7): 122-132.
Ling-shuang ZENG, Pei-ying LI, Zong-jiu SUN, Xiao-fan SUN. Analysis of antioxidant enzyme protection systems and gene expression differences in two Xinjiang bermudagrass genotypes with contrasting drought resistance[J]. Acta Prataculturae Sinica, 2022, 31(7): 122-132.
基因 Gene | 正/反向 Forward/reverse | 引物序列 Primer sequences (5'-3') | 扩增产物大小 Amplication product size (bp) |
|---|---|---|---|
| Cu/Zn-SOD | Forward | AGCGAGCGACTTACAATGGC | 208 |
| Reverse | AACACTGAAGGCGTGGCTGA | ||
| 2-Cys POD | Forward | TTTGATCCCTGATCAGGGCA | 185 |
| Reverse | GCCTGAAGAGTCCTCATGGT | ||
| GPX 4 | Forward | TAAGTGAAGCCAGTATCAGC | 230 |
| Reverse | AGGAAAGCAGATGAGAAGAC | ||
| DHAR | Forward | GCAATGTGCGGGATGAAGAT | 260 |
| Reverse | GCTCCAATGAAACCTGGGCT | ||
| CAT | Forward | CCATGAGATCAAGGCCATCT | 103 |
| Reverse | ATCTTACATGCTCGGCTTGG | ||
| Cyt-APX | Forward | GGTTCAATTAGATACGAGGAAGAGT | 129 |
| Reverse | GGCAAGCTGATGAAGGTCTG | ||
| 内参基因 Actin | Forward | GCTCAACCCCAAGGCTAAC | 186 |
| Reverse | AGAGCGTATCCCTCGTAGATG |
表 1 实时荧光定量引物信息
Table 1 Real-time fluorescence quantitative primer information
基因 Gene | 正/反向 Forward/reverse | 引物序列 Primer sequences (5'-3') | 扩增产物大小 Amplication product size (bp) |
|---|---|---|---|
| Cu/Zn-SOD | Forward | AGCGAGCGACTTACAATGGC | 208 |
| Reverse | AACACTGAAGGCGTGGCTGA | ||
| 2-Cys POD | Forward | TTTGATCCCTGATCAGGGCA | 185 |
| Reverse | GCCTGAAGAGTCCTCATGGT | ||
| GPX 4 | Forward | TAAGTGAAGCCAGTATCAGC | 230 |
| Reverse | AGGAAAGCAGATGAGAAGAC | ||
| DHAR | Forward | GCAATGTGCGGGATGAAGAT | 260 |
| Reverse | GCTCCAATGAAACCTGGGCT | ||
| CAT | Forward | CCATGAGATCAAGGCCATCT | 103 |
| Reverse | ATCTTACATGCTCGGCTTGG | ||
| Cyt-APX | Forward | GGTTCAATTAGATACGAGGAAGAGT | 129 |
| Reverse | GGCAAGCTGATGAAGGTCTG | ||
| 内参基因 Actin | Forward | GCTCAACCCCAAGGCTAAC | 186 |
| Reverse | AGAGCGTATCCCTCGTAGATG |
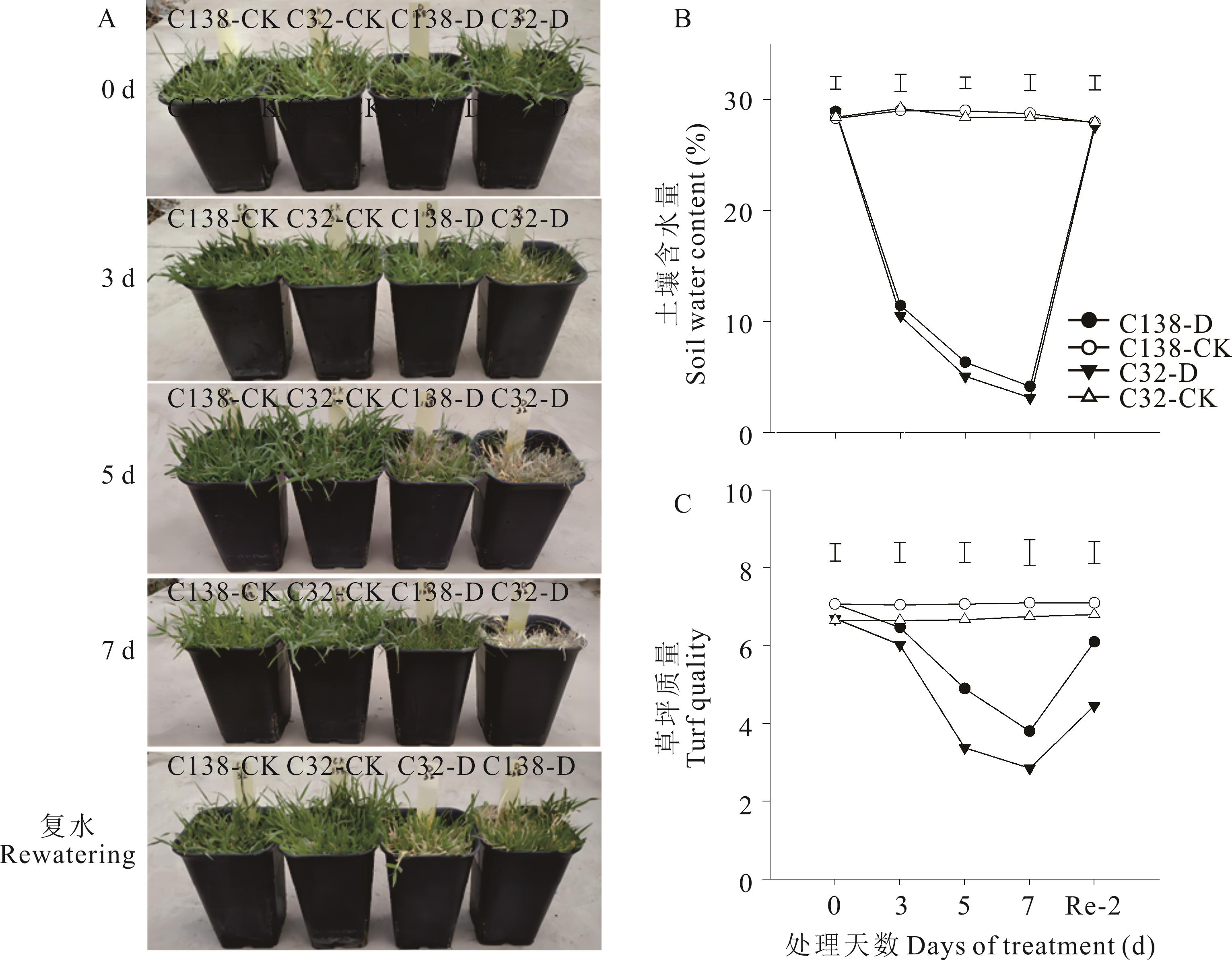
图1 在干旱胁迫和非胁迫条件下,两种不同的耐旱狗牙根基因型的表型(A)、土壤水分(B)和草坪质量(C)的变化竖线表示在采样日之间比较处理之间的最小显著差异值(P<0.05),C138-CK、C32-CK表示C138、C32材料正常浇水处理,C138-D、C32-D表示C138、C32材料干旱-复水处理,Re-2 d表示复水第2天,下同。Vertical bars indicate least significant difference values (P<0.05) for the comparison among treatments at the sampling day. C138-CK and C32-CK indicate normal watering treatment for C138 and C32 materials, and C138-D and C32-D indicate drought-rewatering treatment for C138 and C32 materials, Re-2 means the second day of rewatering. The same below.
Fig.1 Change in the phenotypes (A), soil water content (B) and turf quality (C) of two different drought tolerant bermudagrass genotypes under stressed and non-stressed conditions

图4 干旱胁迫下狗牙根抗氧化酶基因的表达变化1表示C138; 2表示C32; Re表示复水。*表示同一天数不同基因型存在显著差异(P<0.05)。 1 means C138; 2 means C32; Re means rewatering. * indicates that there are significant different drought tolerant bermudagrass at the same day (P<0.05).
Fig.4 Changes of antioxidant enzyme gene expression in bermudagrass under drought stress
| 1 | Zandalinas S I, Mittler R, Balfagon D, et al. Plant adaptations to the combination of drought and high temperatures. Physiologia Plantarum, 2018, 162(1): 1-12. |
| 2 | Fathi A, Tari D B. Effect of drought stress and its mechanism in plants. International Journal of Life Sciences, 2016, 10(1): 1-6. |
| 3 | Cui Y, Wang J, Wang X, et al. Phenotypic and genotypic diversity for drought tolerance among and within perennial ryegrass accessions. HortScience, 2015, 50(8): 1148-1154. |
| 4 | Fang Y, Xiong L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cellular & Molecular Life Sciences, 2014, 72(4): 673-689. |
| 5 | Qian Y L, Fry J. Water relations and drought tolerance of four turfgrasses. Journal of the American Society for Horticultural Science, 1997, 122(1): 129-133. |
| 6 | Husmoen D, Vietor D, Rouquette F M, et al. Variation of responses to water stress between ‘Tifton 85’ and ‘Tifway’ or ‘Coastal’ bermudagrass. Crop Science, 2012, 52(5): 2385-2391. |
| 7 | Sharma P, Jha A, Dubey R S, et al. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012(1): 1-26. |
| 8 | Avramova V, Abd Elgawad H, Vasileva I, et al. High antioxidant activity facilitates maintenance of cell division in leaves of drought tolerant maize hybrids. Frontiers in Plant Science, 2017, 8(1): 1-16. |
| 9 | Prochazkova D, Sairam R K, Srivastava G C, et al. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Science, 2001, 161(4): 765-771. |
| 10 | Huang S, Jiang S, Liang J, et al. Current knowledge of bermudagrass responses to abiotic stresses. Breeding Science, 2019, 69(2): 215-226. |
| 11 | Sharma P, Dubey R S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regulation, 2005, 46(3): 209-221. |
| 12 | Elstner E F. Oxygen activation and oxygen toxicity. Annual Review of Plant Physiology, 2003, 33(1): 73-96. |
| 13 | Huang B, Dacosta M, Jiang Y. Research advances in mechanisms of turfgrass tolerance to abiotic stresses: From physiology to molecular biology. Critical Reviews in Plant Sciences, 2014, 33(2): 141-189. |
| 14 | Xu L, Han L, Huang B. Antioxidant enzyme activities and gene expression patterns in leaves of kentucky bluegrass in response to drought and post-drought recovery. Journal of the American Society for Horticultural Science, 2011, 136(4): 247-255. |
| 15 | Liu J, Li J, Su X, et al. Grafting improves drought tolerance by regulating antioxidant enzyme activities and stress-responsive gene expression in tobacco. Environmental & Experimental Botany, 2014, 107(1): 173-179. |
| 16 | Sanyal R P, Samant A, Prashar V, et al. Biochemical and functional characterization of OsCSD3, a novel CuZn superoxide dismutase from rice. The Biochemical Journal, 2018, 475(19): 3105-3121. |
| 17 | Zhao Y, Du H, Wang Z, et al. Identification of proteins associated with water-deficit tolerance in C4 perennial grass species, Cynodon dactylon×Cynodon transvaalensis and Cynodon dactylon. Physiologia Plantarum, 2011, 141(1): 40-55. |
| 18 | Zeng L S, Li P Y, Sun X F, et al. A multi-trait evaluation of drought resistance of bermudagrass (Cynodon dactylon)germplasm from different habitats in Xinjiang Province. Acta Prataculturae Sinica, 2020, 29(8): 155-169. |
| 曾令霜, 李培英, 孙晓梵, 等. 新疆不同生境狗牙根种质抗旱性综合评价. 草业学报, 2020, 29(8): 155-169. | |
| 19 | Hanson A D, Rathinasabapathi B, Rivoal J, et al. Osmoprotective compounds in the Plumbaginaceae: A natural experiment in metabolic engineering of stress tolerance. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(1): 306-310. |
| 20 | Hu L, Wang Z, Du H, et al. Differential accumulation of dehydrins in response to water stress for hybrid and common bermudagrass genotypes differing in drought tolerance. Journal of Plant Physiology, 2010, 167(2): 103-109. |
| 21 | Sartory D P, Grobbelaar J U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia, 1984, 114(3): 177-187. |
| 22 | Wang Y S, Ding M D, Gu X G, et al. Analysis of interfering substance in the measurement of malondialdehyde content in plant leaves. American Journal of Biochemistry & Biotechnology, 2013, 9(3): 235-242. |
| 23 | André D D A N, José T P, Joaquim E, et al. Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. Journal of Plant Physiology, 2005, 162(10): 1114-1122. |
| 24 | Giannopolitis C N, Ries S K. Superoxide dismutases I. occurrence in higher plants. Plant Physiology, 1977, 59(2): 309-314. |
| 25 | Kochhar S, Kochhar V K, Khanduja S D. Changes in the pattern of isoperoxidases during maturation of grape berries cv. Gulabi as affected by ethephon (2-chloroethyl phosphoric acid). American Journal of Enology and Viticulture, 1979, 30(4): 275-277. |
| 26 | Chance B, Maehly A C. The assay of catalases and peroxidases. Methods in Enzymology, 1955, 2(1): 764-775. |
| 27 | Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant & Cell Physiology, 1981, 22(5): 867-880. |
| 28 | Lv A M, Wang S Y, Zhang J, et al. Expression spectrum analysis of bermudagrass (Cynodon dactylono L.) drought-tolerant gene. Journal of Shanghai Jiaotong University (Agricultural Science), 2015, 33(3): 14-20. |
| 吕爱敏, 王生银, 张菁, 等. 狗牙根耐旱基因的表达谱分析. 上海交通大学学报(农业科学版), 2015, 33(3): 14-20. | |
| 29 | Bian S, Jiang Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Scientia Horticulturae, 2009, 120(2): 267-270. |
| 30 | Kim D W, Rakwal R, Agrawal G K, et al. A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis, 2005, 26(23): 4521-4539. |
| 31 | Mittler R, Vanderauwera S, Gollery M, et al. Reactive oxygen gene network of plants. Trends in Plant Science, 2004, 9(10): 490-498. |
| 32 | Yu J, Sun L, Fan N, et al. Physiological factors involved in positive effects of elevated carbon dioxide concentration on bermudagrass tolerance to salinity stress. Environmental and Experimental Botany, 2015, 115(1): 20-27. |
| 33 | Farooq M, Wahid A, Kobayashi N, et al. Plant drought stress: Effects, mechanisms and management. Agronomy for Sustainable Development, 2009, 29(1): 185-212. |
| 34 | Dacosta M H B. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in responses to drought stress. Journal of the American Society for Horticultural Science, 2007, 132(3): 319-326. |
| 35 | Du H, Zhou P, Huang B. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environmental and Experimental Botany, 2013, 87(1): 159-166. |
| 36 | Bi A, Fan J, Hu Z, et al. Differential acclimation of enzymatic antioxidant metabolism and photosystem II photochemistry in tall fescue under drought and heat and the combined stresses. Frontiers in Plant Science, 2016, 7(1): 1-12. |
| 37 | Fu J, Huang B. Involvement of antioxidant and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environmental & Experimental Botany, 2001, 45(2): 105-114. |
| 38 | Andréia C, Gisele P, Barcellos R S, et al. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genetics & Molecular Biology, 2012, 35, 4(Supple): 1011-1019. |
| 39 | Luo N, Yu X Q, Nie G, et al. Specific peroxidases differentiate Brachypodium distachyon accessions and are associated with drought tolerance traits. Annals of Botany, 2016, 118(2): 259-270. |
| 40 | Zhang X, Ervin E H, Liu Y, et al. Differential responses of antioxidants, abscisic acid, and auxin to deficit irrigation in two perennial ryegrass cultivars contrasting in drought tolerance. Journal of the American Society for Horticultural Science American Society for Horticultural Science, 2015, 140(6): 562-572. |
| 41 | Aydin M, Tombuloglu G, Sakcali M S, et al. Boron alleviates drought stress by enhancing gene expression and antioxidant enzyme activity. Soil Science and Plant Nutrition, 2019, 19(1): 545-555. |
| 42 | Shi H, Ye T, Chan Z. Comparative proteomic responses of two bermudagrass (Cynodon dactylon (L). Pers.) varieties contrasting in drought stress resistance. Plant Physiology & Biochemistry, 2014, 82(1): 218-228. |
| 43 | Zhang M, Jin Z Q, Zhao J, et al. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regulation, 2015, 75(2): 567-574. |
| 44 | Ji Y, Zhang X, Yan P, et al. Osmolyte accumulation, antioxidant enzyme activities and gene expression patterns in leaves of orchardgrass during drought stress and recovery. Grassland Science, 2014, 60(3): 131-141. |
| [1] | 金祎婷, 刘文辉, 刘凯强, 梁国玲, 贾志锋. 全生育期干旱胁迫对‘青燕1号’燕麦叶绿素荧光参数的影响[J]. 草业学报, 2022, 31(6): 112-126. |
| [2] | 苏世平, 李毅, 刘小娥, 种培芳, 单立山, 后有丽. 外源脯氨酸对缓解红砂干旱胁迫的机理研究[J]. 草业学报, 2022, 31(6): 127-138. |
| [3] | 孙晓梵, 张一龙, 李培英, 孙宗玖. 不同施氮量对干旱下狗牙根抗氧化酶活性及渗透调节物质含量的影响[J]. 草业学报, 2022, 31(6): 69-78. |
| [4] | 卫宏健, 丁杰, 张巨明, 杨文, 王咏琪, 刘天增. 践踏胁迫下狗牙根草坪土壤真菌群落结构的变化特征[J]. 草业学报, 2022, 31(4): 102-112. |
| [5] | 任雪锋, 邓亚博, 臧国长, 郑轶琦. 基于SSR标记的河南省狗牙根遗传多样性及群体遗传结构分析[J]. 草业学报, 2022, 31(3): 60-70. |
| [6] | 王志恒, 魏玉清, 赵延蓉, 王悦娟. 基于转录组学比较研究甜高粱幼苗响应干旱和盐胁迫的生理特征[J]. 草业学报, 2022, 31(3): 71-84. |
| [7] | 赵利清, 郝志刚, 崔笑岩, 彭向永. 赤霉素及其抑制剂调控草地早熟禾生长及赤霉素相关基因表达的研究[J]. 草业学报, 2022, 31(3): 85-91. |
| [8] | 高鹏飞, 张静, 范卫芳, 高冰, 郝宏娟, 吴建慧. 干旱胁迫对光叉委陵菜根系特征、结构和生理特性的影响[J]. 草业学报, 2022, 31(2): 203-212. |
| [9] | 魏娜, 李艳鹏, 马艺桐, 刘文献. 全基因组水平紫花苜蓿TCP基因家族的鉴定及其在干旱胁迫下表达模式分析[J]. 草业学报, 2022, 31(1): 118-130. |
| [10] | 赵欣桐, 陈晓东, 李子吉, 张巨明, 刘天增. 植物内生肠杆菌对狗牙根耐盐性的调控研究[J]. 草业学报, 2021, 30(9): 127-136. |
| [11] | 赵颖, 辛夏青, 魏小红. 一氧化氮对干旱胁迫下紫花苜蓿氮代谢的影响[J]. 草业学报, 2021, 30(9): 86-96. |
| [12] | 臧真凤, 白婕, 刘丛, 昝看卓, 龙明秀, 何树斌. 紫花苜蓿形态和生理指标响应干旱胁迫的品种特异性[J]. 草业学报, 2021, 30(6): 73-81. |
| [13] | 罗巧玉, 王彦龙, 陈志, 马永贵, 任启梅, 马玉寿. 水分逆境对发草脯氨酸及其代谢途径的影响[J]. 草业学报, 2021, 30(5): 75-83. |
| [14] | 候怡谣, 李霄, 龙瑞才, 杨青川, 康俊梅, 郭长虹. 过量表达紫花苜蓿MsHB7基因对拟南芥耐旱性的影响[J]. 草业学报, 2021, 30(4): 170-179. |
| [15] | 刘凯强, 刘文辉, 贾志锋, 梁国玲, 马祥. 干旱胁迫对‘青燕1号’燕麦产量及干物质积累与分配的影响[J]. 草业学报, 2021, 30(3): 177-188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||