

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2023, Vol. 32 ›› Issue (7): 61-71.DOI: 10.11686/cyxb2022348
管瑾1( ), 郭一荻1, 刘凌云1, 尹淑霞1(
), 郭一荻1, 刘凌云1, 尹淑霞1( ), 滕珂2(
), 滕珂2( )
)
收稿日期:2022-08-30
修回日期:2022-10-07
出版日期:2023-07-20
发布日期:2023-05-26
通讯作者:
尹淑霞,滕珂
作者简介:E-mail: tengke.123@163.com基金资助:
Jin GUAN1( ), Yi-di GUO1, Ling-yun LIU1, Shu-xia YIN1(
), Yi-di GUO1, Ling-yun LIU1, Shu-xia YIN1( ), Ke TENG2(
), Ke TENG2( )
)
Received:2022-08-30
Revised:2022-10-07
Online:2023-07-20
Published:2023-05-26
Contact:
Shu-xia YIN,Ke TENG
摘要:
为了结合转基因方法探索结缕草基因功能,建立高效、快速的结缕草原生质体制备及瞬时基因表达系统,利用正交试验对影响原生质体制备的主要因素进行优化,同时利用原生质体进行亚细胞定位和蛋白互作研究。结果表明,当酶配比为2% (w/v) cellulase R10和1.5% (w/v) macerozyme R10,酶解时间为7 h时,原生质体产量和活性均达到最高,分别为1.23×107个·mL-1和98%以上,满足后续转化所需。多次重复试验表明,加入5~10 μg质粒,原生质体转化率可达75%以上。利用原生质体转化体系进行了亚细胞定位检测,结果发现结缕草ZjNOL和ZjNYC1定位在叶绿体中,ZjZFN定位在细胞核中。双分子荧光互补分析证明ZjNOL和ZjNYC1在叶绿体中相互作用。原生质体制备及转化方法与遗传学和组学技术相结合,能够为结缕草基因功能研究和基因编辑提供支持。
管瑾, 郭一荻, 刘凌云, 尹淑霞, 滕珂. 结缕草叶肉细胞原生质体瞬时基因表达系统的构建[J]. 草业学报, 2023, 32(7): 61-71.
Jin GUAN, Yi-di GUO, Ling-yun LIU, Shu-xia YIN, Ke TENG. An efficient protocol for Zoysia japonica mesophyll protoplast isolation and transformation, and its application in subcellular localization and protein interaction analysis[J]. Acta Prataculturae Sinica, 2023, 32(7): 61-71.
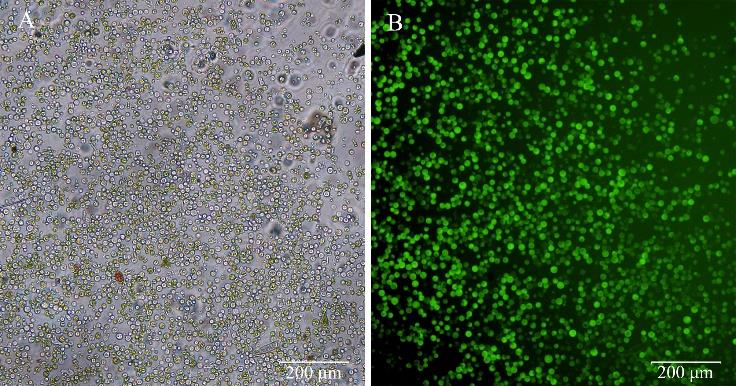
图1 结缕草原生质体制备情况A:光学显微镜下原生质体的形态特征Morphological characteristics of protoplasts observed by light microscopy;B: 光学显微镜下FDA染色后的原生质体形态特征Morphological characteristics of protoplasts stained with FDA, observed by light microscopy.
Fig.1 Isolation of mesophyll protoplasts from Z. japonica
| 引物名称Primer name | 序列Sequence (5'-3') |
|---|---|
| ZjNYC1-F | CTCGTCCCGACCTTATCCGC |
| ZjNYC1-R | GGGCACGGGGTCATCCAG |
| ZjNOL-F | CTCCACACAAGACTCCATTCG |
| ZjNOL-R | GAAAAAGGAATGGTTCAAAACAGAT |
| ZjZFN-F | ATGTCGTCCGCCATGGAATT |
| ZjZFN-R | TCACGCGGTCATGAGGAGGC |
| 3302Y3-ZjNYC1-F | cctactagtcctagggacgtcaATGGCCGCCGCCGTCGCGCA |
| 3302Y3-ZjNYC1-R | tgctcaccatacgcgttacagaTGTGCCTGGAAGAGGACCAC |
| 3302Y3-ZjNOL-F | cctactagtcctagggacgtcaATGGCTGCCAGCGTCAGCATCG |
| 3302Y3-ZjNOL-R | tgctcaccatacgcgttacagaATCTTCAACAACATACTTAT |
| 3302Y3-ZjZFN-F | cacgggggactcttgaccatggtaATGTCGTCCGCCATGGAATT |
| 3302Y3-ZjZFN-R | ggtacacgcgtactagtcagatcCGCGGTCATGAGGAGGCGGG |
| 35S-pSPY-ZjNYC1-F | caggcctggcgcgccactagtgATGGCCGCCGCCGTCGCGCA |
| 35S-pSPY-ZjNYC1-R | ggtcgacagtactatcgatggaTGTGCCTGGAAGAGGACCAC |
| 35S-pSPY-ZjNOL-F | caggcctggcgcgccactagtgATGGCTGCCAGCGTCAGCATCG |
| 35S-pSPY-ZjNOL-R | ggtcgacagtactatcgatggaATCTTCAACAACATACTTAT |
表1 用于基因克隆、表达分析和载体构建的引物
Table 1 Primers used for gene cloning, expression analysis and plasmids construction
| 引物名称Primer name | 序列Sequence (5'-3') |
|---|---|
| ZjNYC1-F | CTCGTCCCGACCTTATCCGC |
| ZjNYC1-R | GGGCACGGGGTCATCCAG |
| ZjNOL-F | CTCCACACAAGACTCCATTCG |
| ZjNOL-R | GAAAAAGGAATGGTTCAAAACAGAT |
| ZjZFN-F | ATGTCGTCCGCCATGGAATT |
| ZjZFN-R | TCACGCGGTCATGAGGAGGC |
| 3302Y3-ZjNYC1-F | cctactagtcctagggacgtcaATGGCCGCCGCCGTCGCGCA |
| 3302Y3-ZjNYC1-R | tgctcaccatacgcgttacagaTGTGCCTGGAAGAGGACCAC |
| 3302Y3-ZjNOL-F | cctactagtcctagggacgtcaATGGCTGCCAGCGTCAGCATCG |
| 3302Y3-ZjNOL-R | tgctcaccatacgcgttacagaATCTTCAACAACATACTTAT |
| 3302Y3-ZjZFN-F | cacgggggactcttgaccatggtaATGTCGTCCGCCATGGAATT |
| 3302Y3-ZjZFN-R | ggtacacgcgtactagtcagatcCGCGGTCATGAGGAGGCGGG |
| 35S-pSPY-ZjNYC1-F | caggcctggcgcgccactagtgATGGCCGCCGCCGTCGCGCA |
| 35S-pSPY-ZjNYC1-R | ggtcgacagtactatcgatggaTGTGCCTGGAAGAGGACCAC |
| 35S-pSPY-ZjNOL-F | caggcctggcgcgccactagtgATGGCTGCCAGCGTCAGCATCG |
| 35S-pSPY-ZjNOL-R | ggtcgacagtactatcgatggaATCTTCAACAACATACTTAT |
因素 Factors | Cellulase R10 (%, w/v) | Macerozyme R10 (%, w/v) | 酶解时间 Enzymolysis time (h) |
|---|---|---|---|
| 1 | 1 | 0.5 | 4 |
| 2 | 2 | 1.0 | 5 |
| 3 | 3 | 1.5 | 6 |
| 4 | 4 | 2.0 | 7 |
表2 原生质体制备酶配比和酶解时间
Table 2 The enzyme ratio and enzymolysis time in the protoplast isolation experiment
因素 Factors | Cellulase R10 (%, w/v) | Macerozyme R10 (%, w/v) | 酶解时间 Enzymolysis time (h) |
|---|---|---|---|
| 1 | 1 | 0.5 | 4 |
| 2 | 2 | 1.0 | 5 |
| 3 | 3 | 1.5 | 6 |
| 4 | 4 | 2.0 | 7 |
溶液名称 Solution name | 溶液配方 Solution composition | 储存条件 Storage | 用途 Usage |
|---|---|---|---|
| 酶解液Enzyme solution | cellulase R10,macerozyme R10,甘露醇Mannitol,10 mmol·L-1 MES,pH 5.7,10 mmol·L-1 CaCl2,0.1% (w/v) BSA | 现用现配,室温Freshly prepared, room temperature | 制备原生质体Preparation of protoplasts |
| W5溶液 W5 solution | 154 mmol·L-1 NaCl,125 mmol·L-1 CaCl2,5 mmol·L-1 KCl,2 mmol·L-1 MES,pH 5.7 | 4 ℃ | 释放和清洗原生质体Release and wash protoplasts |
| MMG溶液 MMG solution | 0.4 mol·L-1 甘露醇Mannitol, 15 mmol·L-1 MgCl2, 4 mmol·L-1 MES,pH 5.7 | 4 ℃ | 重悬原生质体Resuspend protoplasts |
| PEG溶液 PEG solution | 40% (w/v) PEG 4000, 0.2 mol·L-1 甘露醇Mannitol,0.1 mol·L-1 CaCl2 | 现用现配,室温Freshly prepared, room temperature | 介导原生质体转化Transform plasmids into protoplasts |
| WI溶液 WI solution | 0.5 mol·L-1 甘露醇Mannitol,20 mmol·L-1 KCl,4 mmol·L-1 MES,pH 5.7 | 4 ℃ | 重悬转化体系Resuspend transformation system |
表3 原生质体制备和转化的溶液配方
Table 3 Solution recipes for protoplast isolation and transformation
溶液名称 Solution name | 溶液配方 Solution composition | 储存条件 Storage | 用途 Usage |
|---|---|---|---|
| 酶解液Enzyme solution | cellulase R10,macerozyme R10,甘露醇Mannitol,10 mmol·L-1 MES,pH 5.7,10 mmol·L-1 CaCl2,0.1% (w/v) BSA | 现用现配,室温Freshly prepared, room temperature | 制备原生质体Preparation of protoplasts |
| W5溶液 W5 solution | 154 mmol·L-1 NaCl,125 mmol·L-1 CaCl2,5 mmol·L-1 KCl,2 mmol·L-1 MES,pH 5.7 | 4 ℃ | 释放和清洗原生质体Release and wash protoplasts |
| MMG溶液 MMG solution | 0.4 mol·L-1 甘露醇Mannitol, 15 mmol·L-1 MgCl2, 4 mmol·L-1 MES,pH 5.7 | 4 ℃ | 重悬原生质体Resuspend protoplasts |
| PEG溶液 PEG solution | 40% (w/v) PEG 4000, 0.2 mol·L-1 甘露醇Mannitol,0.1 mol·L-1 CaCl2 | 现用现配,室温Freshly prepared, room temperature | 介导原生质体转化Transform plasmids into protoplasts |
| WI溶液 WI solution | 0.5 mol·L-1 甘露醇Mannitol,20 mmol·L-1 KCl,4 mmol·L-1 MES,pH 5.7 | 4 ℃ | 重悬转化体系Resuspend transformation system |

图3 正交试验A:原生质体产量Protoplast yield;B: 原生质体活性Protoplast activity;C:有效原生质体产量Active protoplast yield. 不同小写字母表示处理间差异显著(P<0.05)。Different lowercase lerrers indicate significant differernce among different treatments (P<0.05).
Fig.3 The results of the orthogonal experiment
处理编号 Treatment No. | Cellulase R10 | Macerozyme R10 | 酶解时间 Enzymolysis time (h) | 原生质体产量 Protoplast yield (×106 个protoplasts·mL-1) | 原生质体活性 Protoplast activity (%) | 有效原生质体产量 Active protoplast yield (×106 个protoplasts·mL-1) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 2.25 | 78.03 | 1.76 |
| 2 | 1 | 2 | 2 | 2.51 | 81.64 | 2.05 |
| 3 | 1 | 3 | 3 | 4.05 | 86.13 | 3.49 |
| 4 | 1 | 4 | 4 | 4.54 | 85.25 | 3.87 |
| 5 | 2 | 1 | 2 | 6.54 | 85.25 | 5.58 |
| 6 | 2 | 2 | 1 | 3.91 | 84.14 | 3.28 |
| 7 | 2 | 3 | 4 | 12.25 | 98.87 | 12.11 |
| 8 | 2 | 4 | 3 | 7.81 | 88.07 | 6.87 |
| 9 | 3 | 1 | 3 | 6.03 | 81.84 | 4.94 |
| 10 | 3 | 2 | 4 | 7.08 | 84.56 | 5.99 |
| 11 | 3 | 3 | 1 | 4.47 | 88.31 | 3.94 |
| 12 | 3 | 4 | 2 | 6.63 | 84.71 | 5.62 |
| 13 | 4 | 1 | 4 | 6.43 | 81.29 | 5.23 |
| 14 | 4 | 2 | 3 | 6.08 | 85.16 | 5.18 |
| 15 | 4 | 3 | 2 | 6.63 | 90.11 | 5.97 |
| 16 | 4 | 4 | 1 | 5.25 | 85.94 | 4.52 |
原生质体产量 Protoplast yield (×106个protoplasts·mL-1) | K1 | 3.34 | 5.32 | 3.97 | ||
| K2 | 7.63 | 4.90 | 5.58 | |||
| K3 | 6.05 | 6.85 | 5.99 | |||
| K4 | 6.10 | 6.06 | 7.58 | |||
| 范围Range | 4.29 | 1.53 | 3.61 | |||
| 排序Rank | cellulase R10>酶解时间Enzymolysis time>macerozyme R10 | |||||
原生质体活性 Protoplast activity (%) | K1 | 82.76 | 81.60 | 84.10 | ||
| K2 | 89.08 | 83.87 | 85.43 | |||
| K3 | 84.85 | 90.85 | 85.30 | |||
| K4 | 85.62 | 85.99 | 87.49 | |||
| 范围Range | 6.32 | 9.25 | 2.07 | |||
| 排序Rank | macerozyme R10>cellulase R10>酶解时间Enzymolysis time | |||||
有效原生质体产量 Active protoplast yield (×106个protoplasts·mL-1) | K1 | 2.79 | 4.38 | 3.38 | ||
| K2 | 6.96 | 4.13 | 4.80 | |||
| K3 | 5.12 | 6.38 | 5.12 | |||
| K4 | 5.23 | 5.22 | 6.80 | |||
| 范围Range | 4.17 | 2.00 | 3.42 | |||
| 排序Rank | cellulase R10>酶解时间Enzymolysis time>macerozyme R10 | |||||
表4 影响原生质体制备效率的三因素正交试验
Table 4 Orthogonal experiment of three factors affecting the efficiency of protoplast isolation
处理编号 Treatment No. | Cellulase R10 | Macerozyme R10 | 酶解时间 Enzymolysis time (h) | 原生质体产量 Protoplast yield (×106 个protoplasts·mL-1) | 原生质体活性 Protoplast activity (%) | 有效原生质体产量 Active protoplast yield (×106 个protoplasts·mL-1) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 2.25 | 78.03 | 1.76 |
| 2 | 1 | 2 | 2 | 2.51 | 81.64 | 2.05 |
| 3 | 1 | 3 | 3 | 4.05 | 86.13 | 3.49 |
| 4 | 1 | 4 | 4 | 4.54 | 85.25 | 3.87 |
| 5 | 2 | 1 | 2 | 6.54 | 85.25 | 5.58 |
| 6 | 2 | 2 | 1 | 3.91 | 84.14 | 3.28 |
| 7 | 2 | 3 | 4 | 12.25 | 98.87 | 12.11 |
| 8 | 2 | 4 | 3 | 7.81 | 88.07 | 6.87 |
| 9 | 3 | 1 | 3 | 6.03 | 81.84 | 4.94 |
| 10 | 3 | 2 | 4 | 7.08 | 84.56 | 5.99 |
| 11 | 3 | 3 | 1 | 4.47 | 88.31 | 3.94 |
| 12 | 3 | 4 | 2 | 6.63 | 84.71 | 5.62 |
| 13 | 4 | 1 | 4 | 6.43 | 81.29 | 5.23 |
| 14 | 4 | 2 | 3 | 6.08 | 85.16 | 5.18 |
| 15 | 4 | 3 | 2 | 6.63 | 90.11 | 5.97 |
| 16 | 4 | 4 | 1 | 5.25 | 85.94 | 4.52 |
原生质体产量 Protoplast yield (×106个protoplasts·mL-1) | K1 | 3.34 | 5.32 | 3.97 | ||
| K2 | 7.63 | 4.90 | 5.58 | |||
| K3 | 6.05 | 6.85 | 5.99 | |||
| K4 | 6.10 | 6.06 | 7.58 | |||
| 范围Range | 4.29 | 1.53 | 3.61 | |||
| 排序Rank | cellulase R10>酶解时间Enzymolysis time>macerozyme R10 | |||||
原生质体活性 Protoplast activity (%) | K1 | 82.76 | 81.60 | 84.10 | ||
| K2 | 89.08 | 83.87 | 85.43 | |||
| K3 | 84.85 | 90.85 | 85.30 | |||
| K4 | 85.62 | 85.99 | 87.49 | |||
| 范围Range | 6.32 | 9.25 | 2.07 | |||
| 排序Rank | macerozyme R10>cellulase R10>酶解时间Enzymolysis time | |||||
有效原生质体产量 Active protoplast yield (×106个protoplasts·mL-1) | K1 | 2.79 | 4.38 | 3.38 | ||
| K2 | 6.96 | 4.13 | 4.80 | |||
| K3 | 5.12 | 6.38 | 5.12 | |||
| K4 | 5.23 | 5.22 | 6.80 | |||
| 范围Range | 4.17 | 2.00 | 3.42 | |||
| 排序Rank | cellulase R10>酶解时间Enzymolysis time>macerozyme R10 | |||||
指标 Index | 原生质体产量 Protoplast yield | 原生质体活性 Protoplast activity |
|---|---|---|
| 原生质体活性Protoplast activity | 0.774 | |
有效原生质体产量 Active protoplast yield | 0.993 | 0.830 |
表5 原生质体制备中各因素的相关性
Table 5 The correlation between the indexes of protoplast isolation
指标 Index | 原生质体产量 Protoplast yield | 原生质体活性 Protoplast activity |
|---|---|---|
| 原生质体活性Protoplast activity | 0.774 | |
有效原生质体产量 Active protoplast yield | 0.993 | 0.830 |
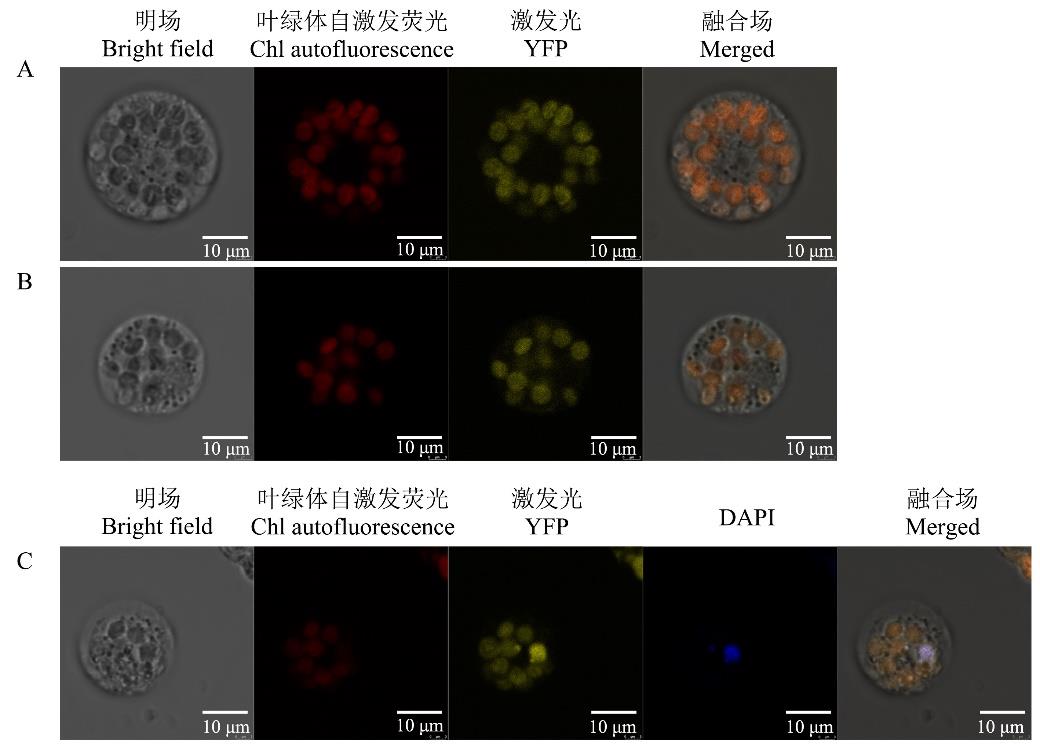
图4 不同载体在结缕草原生质体中的亚细胞定位A:ZjNOL-YFP;B: ZjNYC1-YFP;C:ZjZFN-YFP; DAPI:二盐酸-4’,6-二脒基-2-苯基吲哚4’,6-diamidino-2-phenylindole dihydrochloride.
Fig.4 Subcellular localization for different vector in Z. japonica protoplasts
| 1 | Ueki S, Lacroix B, Krichevsky A, et al. Functional transient genetic transformation of Arabidopsis leaves by biolistic bombardment. Nature Protocols, 2009, 4(1): 71-77. |
| 2 | Song G Q, Sink K C. Optimizing shoot regeneration and transient expression factors for Agrobacterium tumefaciens transformation of sour cherry (Prunus cerasus L.) cultivar Montmorency. Scientia Horticulturae, 2005, 106(1): 60-69. |
| 3 | Manavella P A, Chan R L. Transient transformation of sunflower leaf discs via an Agrobacterium-mediated method: Applications for gene expression and silencing studies. Nature Protocols, 2009, 4(11): 1699-1707. |
| 4 | Andrieu A, Breitler J C, Siré C, et al. An in planta, Agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (Oryza sativa L.) leaves. Rice, 2012, 5(1): 1-12. |
| 5 | Yoo S, Cho Y, Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols, 2007, 2: 1565-1572. |
| 6 | Cao J, Yao D, Lin F, et al. PEG-mediated transient gene expression and silencing system in maize mesophyll protoplasts: A valuable tool for signal transduction study in maize. Acta Physiologiae Plantarum, 2014, 36(5): 1271-1281. |
| 7 | Yu C, Wang L, Chen C, et al. Protoplast: A more efficient system to study nucleo-cytoplasmic interactions. Biochemical and Biophysical Research Communications, 2014, 450(4): 1575-1580. |
| 8 | Zhang Y, Su J, Duan S, et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods, 2011, 7(1): 30. |
| 9 | Yu G, Cheng Q, Xie Z, et al. An efficient protocol for perennial ryegrass mesophyll protoplast isolation and transformation, and its application on interaction study between LpNOL and LpNYC1. Plant Methods, 2017, 13(1): 46. |
| 10 | Ma W, Yi F, Xiao Y, et al. Isolation of leaf mesophyll protoplasts optimized by orthogonal design for transient gene expression in Catalpa bungei. Scientia Horticulturae, 2020, 274: 109684. |
| 11 | Rezazadeh R, Niedz R P. Protoplast isolation and plant regeneration of guava (Psidium guajava L.) using experiments in mixture-amount design. Plant Cell, Tissue and Organ Culture (PCTOC), 2015, 122(3): 585-604. |
| 12 | Yao L, Liao X, Gan Z, et al. Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.). Scientia Horticulturae, 2016, 209: 14-21. |
| 13 | Iguti S. Effects of mannitol on protoplasts of Saccharomyces cerevisiae. Plant and Cell Physiology, 1968, 9(3): 573-576. |
| 14 | Hong S Y, Seo P J, Cho S H, et al. Preparation of leaf mesophyll protoplasts for transient gene expression in Brachypodium distachyon. Journal of Plant Biology, 2012, 55(5): 390-397. |
| 15 | Wang H, Wang W, Zhan J, et al. An efficient PEG-mediated transient gene expression system in grape protoplasts and its application in subcellular localization studies of flavonoids biosynthesis enzymes. Scientia Horticulturae, 2015, 191: 82-89. |
| 16 | Wang H, Wang W, Li H, et al. Expression and tissue and subcellular localization of anthocyanidin synthase (ANS) in grapevine. Protoplasma, 2011, 248(2): 267-279. |
| 17 | Tan B, Xu M, Chen Y, et al. Transient expression for functional gene analysis using Populus protoplasts. Plant Cell, Tissue and Organ Culture (PCTOC), 2013, 114(1): 11-18. |
| 18 | Weinthal D, Tzfira T. Imaging protein-protein interactions in plant cells by bimolecular fluorescence complementation assay. Trends in Plant Science, 2009, 14(2): 59-63. |
| 19 | Pitzschke A, Persak H. Poinsettia protoplasts-a simple, robust and efficient system for transient gene expression studies. Plant Methods, 2012, 8(1): 14. |
| 20 | Sheen J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiology, 2001, 127(4): 1466-1475. |
| 21 | De Sutter V, Vanderhaeghen R, Tilleman S, et al. Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant Journal, 2005, 44(6): 1065-1076. |
| 22 | Bargmann B O, Marshall-Colon A, Efroni I, et al. TARGET: A transient transformation system for genome-wide transcription factor target discovery. Molecular Plant, 2013, 6(3): 978. |
| 23 | Ping H, Libo S, Jen S. The use of protoplasts to study innate immune responses. Methods in Molecular Biology (Clifton, NJ), 2007, 354: 1-9. |
| 24 | Zong Y, Song Q, Li C, et al. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nature Biotechnology, 2018, 36(10): 950-953. |
| 25 | Rathnam C, Chollet R. Photosynthetic and photorespiratory carbon metabolism in mesophyll protoplasts and chloroplasts isolated from isogenic diploid and tetraploid cultivars of ryegrass (Lolium perenne L.). Plant Physiology, 1980, 65(3): 489-494. |
| 26 | Yanagisawa S, Sheen J. Involvement of maize Dof zinc finger proteins in tissue-specific and light-regulated gene expression. Plant Cell, 1998, 10(1): 75-89. |
| 27 | Augustynowicz J, Lekka M, Burda K, et al. Correlation between chloroplast motility and elastic properties of tobacco mesophyll protoplasts. Acta Physiologiae Plantarum, 2001, 23(3): 291-302. |
| 28 | Augustynowicz J, Krzeszowiec W, Gabrys H. Acquisition of plastid movement responsiveness to light during mesophyll cell differentiation. International Journal of Developmental Biology, 2009, 53(1): 121-127. |
| 29 | Locatelli F, Vannini C, Magnani E, et al. Efficiency of transient transformation in tobacco protoplasts is independent of plasmid amount. Plant Cell Reports, 2003, 21(9): 865-871. |
| 30 | Tanaka H, Hirakawa H, Kosugi S, et al. Sequencing and comparative analyses of the genomes of zoysiagrasses. DNA Research, 2016, 23(2): 171-180. |
| 31 | Guan J, Teng K, Yue Y, et al. Zoysia japonica chlorophyll b reductase gene NOL participates in chlorophyll degradation and photosynthesis. Frontiers in Plant Science, 2022, 13(2022): 906018. |
| 32 | Teng K, Tan P, Guan J, et al. Functional characterization of the chlorophyll b reductase gene NYC1 associated with chlorophyll degradation and photosynthesis in Zoysia japonica. Environmental and Experimental Botany, 2021, 191(2021): 104607. |
| 33 | Marion J, Bach L, Bellec Y, et al. Systematic analysis of protein subcellular localization and interaction using high‐throughput transient transformation of Arabidopsis seedlings. Plant Journal, 2008, 56(1): 169-179. |
| 34 | Teng K, Tan P, Guo W, et al. Heterologous expression of a novel Zoysia japonica C2H2 zinc finger gene, ZjZFN1, improved salt tolerance in Arabidopsis. Frontiers in Plant Science, 2018, 9(2018): 1159. |
| 35 | Guan J, Yin S, Yue Y, et al. Single-molecule long-read sequencing analysis improves genome annotation and sheds new light on the transcripts and splice isoforms of Zoysia japonica. BMC Plant Biology, 2022, 22(1): 1-17. |
| 36 | Jia N, Liu X, Gao H. A DNA2 homolog is required for DNA damage repair, cell cycle regulation, and meristem maintenance in plants. Plant Physiology, 2016, 171(1): 318-333. |
| 37 | Larkin P J. Purification and viability determinations of plant protoplasts. Planta, 1976, 128(3): 213-216. |
| 38 | Guo J, Morrell-Falvey J L, Labbé J L, et al. Highly efficient isolation of Populus mesophyll protoplasts and its application in transient expression assays. PLoS ONE, 2012, 7(9): e44908. |
| 39 | Patton A J, Reicher Z J. Zoysiagrass species and genotypes differ in their winter injury and freeze tolerance. Crop Science, 2007, 47(4): 1619-1627. |
| 40 | Sakuraba Y, Schelbert S, ParkS Y, et al. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell, 2012, 24(2): 507-518. |
| 41 | Sato Y, Morita R, Kusaba S, et al. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant Journal, 2010, 57(1): 120-131. |
| [1] | 姚佳明, 郝欢欢, 张敬, 徐彬. tRNA-sgRNA/Cas9系统介导多年生黑麦草原生质体的基因编辑[J]. 草业学报, 2023, 32(4): 129-141. |
| [2] | 姚佳明, 何悦, 郝欢欢, 黄心如, 张敬, 徐彬. 多年生黑麦草LpPIL5基因特征分析及转录调控[J]. 草业学报, 2022, 31(9): 155-167. |
| [3] | 汪智宇, 李莹, 刘金平, 伍德, 苟蓉. 高温冲击对受丝茅入侵的细叶结缕草现实和潜在竞争力的影响[J]. 草业学报, 2019, 28(8): 106-118. |
| [4] | 滕珂, 张蕊, 檀鹏辉, 岳跃森, 范希峰, 武菊英. 日本结缕草ZjERF1的克隆、转录激活活性、亚细胞定位及表达分析[J]. 草业学报, 2019, 28(6): 56-65. |
| [5] | 姜红岩, 滕珂, 檀鹏辉, 尹淑霞. 日本结缕草ZjZFN1基因对拟南芥的转化及其耐旱性分析[J]. 草业学报, 2019, 28(4): 129-138. |
| [6] | 曾晓琳, 李莹, 刘金平, 游明鸿, 黄曦叶, 黄柳. 干旱对细叶结缕草和入侵杂草丝茅的竞争、生长及抗旱性的影响[J]. 草业学报, 2019, 28(11): 46-59. |
| [7] | 汪智宇, 李莹, 刘金平, 杨小琴, 何林江. 不同修剪频次和丝茅入侵量对细叶结缕草竞争力和草坪质量的影响[J]. 草业学报, 2019, 28(10): 53-65. |
| [8] | 强治全, 杨文博, 张帅, 于正阳, 史学英, 王鑫, 朱维宁, 张林生. 异源表达WZY2-1基因提高拟南芥植株抗旱性[J]. 草业学报, 2018, 27(6): 92-99. |
| [9] | 武志刚, 武舒佳, 王迎春, 郑琳琳. 植物中钙依赖蛋白激酶(CDPK)的研究进展[J]. 草业学报, 2018, 27(1): 204-214. |
| [10] | 祁泽文, 孙鑫博, 樊波, 张雪, 袁建波, 韩烈保. PEG介导的柳枝稷叶肉细胞原生质体瞬时表达体系的建立[J]. 草业学报, 2017, 26(9): 113-120. |
| [11] | 史经昂, 张兵, 肖晓琳, 马晶晶, 杨向阳, 刘建秀. 结缕草肉桂醇脱氢酶基因家族全基因组序列鉴定和表达分析[J]. 草业学报, 2017, 26(6): 111-119. |
| [12] | 檀鹏辉, 袁丽丽, 樊波, 于安东, 董笛, 滕珂, 晁跃辉. 日本结缕草滞绿基因 ZjSGR 对烟草的转化及功能分析[J]. 草业学报, 2017, 26(5): 155-162. |
| [13] | 张雪, 孙鑫博, 樊波, 张胤冰, 韩烈保, 许立新. 结缕草ZjCSD基因的克隆及表达分析[J]. 草业学报, 2017, 26(2): 102-110. |
| [14] | 董笛, 滕珂, 于安东, 檀鹏辉, 梁小红, 韩烈保. 沟叶结缕草八氢番茄红素基因ZmPSY的克隆、亚细胞定位及表达分析[J]. 草业学报, 2017, 26(11): 69-76. |
| [15] | 宋华伟, 刘颖, 王宸, 刘天增, 张巨明. 不同坪床基质物理性质变化及对兰引Ⅲ号结缕草生长的影响[J]. 草业学报, 2016, 25(7): 177-185. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||