

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2022, Vol. 31 ›› Issue (10): 122-134.DOI: 10.11686/cyxb2022082
• 研究论文 • 上一篇
林涛1,2( ), 张立娇1(
), 张立娇1( ), 韩蓉蓉1, 玉永雄1, 蒋曹德1(
), 韩蓉蓉1, 玉永雄1, 蒋曹德1( )
)
收稿日期:2022-02-19
修回日期:2022-04-18
出版日期:2022-10-20
发布日期:2022-09-14
通讯作者:
蒋曹德
作者简介:E-mail: jcdpjx@swu.edu.cn基金资助:
Tao LIN1,2( ), Li-jiao ZHANG1(
), Li-jiao ZHANG1( ), Rong-rong HAN1, Yong-xiong YU1, Cao-de JIANG1(
), Rong-rong HAN1, Yong-xiong YU1, Cao-de JIANG1( )
)
Received:2022-02-19
Revised:2022-04-18
Online:2022-10-20
Published:2022-09-14
Contact:
Cao-de JIANG
摘要:
4CL(4-coumarate: coenzyme A ligase)是木质素合成途径关键酶,已被证明在生物和非生物胁迫、机械损伤抗性等生物过程中具有重要作用,但与柠檬酸分泌相关的耐铝功能还没有报道。本研究选择丹波黑大豆Gm4CL2,利用RT-PCR技术克隆其全长编码序列,蛋白质序列多重序列比对和进化树分析不同物种间的亲缘关系,农杆菌介导浸花法和叶盘法分别遗传转化拟南芥和紫花苜蓿,qRT-PCR技术检测基因的表达水平。序列分析结果发现,Gm4CL2全长编码序列为1668 bp,该基因编码555个氨基酸,为双子叶植物Ⅰ类 4CL。Real-time PCR结果显示,50 μmol·L-1 AlCl3 (pH 4.5)特异诱导Gm4CL2在丹波黑大豆幼苗0~2 cm的根尖组织表达;过表达Gm4CL2拟南芥,在铝处理条件下其根尖AtMATE、AtSTAR1和AtSTAR2表达量显著上调(P<0.05)。Al3+胁迫条件下,过表达Gm4CL2拟南芥根相对伸长量、根尖SOD、POD活性和柠檬酸分泌量显著高于野生型,根尖伊文思蓝和铬天青S染色以及Al3+、ROS、MDA含量显著低于野生型(P<0.05);过表达Gm4CL2紫花苜蓿根相对伸长率、根尖柠檬酸分泌量和生物量显著高于野生型,根尖Al3+含量、伊文思蓝染色显著低于野生型(P<0.05)。细胞壁成分分析表明,Al3+胁迫和非胁迫下,过表达Gm4CL2拟南芥根尖果胶、咖啡酸、阿魏酸比野生型显著降低(P<0.05);Al3+胁迫下,过表达Gm4CL2拟南芥根尖木质素含量显著降低,但是4-香豆酸含量显著升高(P<0.05)。上述结果表明,Gm4CL2为耐铝基因,该基因通过促进细胞壁修饰和柠檬酸分泌提高拟南芥和紫花苜蓿的耐铝性。
林涛, 张立娇, 韩蓉蓉, 玉永雄, 蒋曹德. Gm4CL2基因对拟南芥和紫花苜蓿耐铝性的影响[J]. 草业学报, 2022, 31(10): 122-134.
Tao LIN, Li-jiao ZHANG, Rong-rong HAN, Yong-xiong YU, Cao-de JIANG. Effects of the Gm4CL2 gene on aluminum tolerance of Arabidopsis and alfalfa[J]. Acta Prataculturae Sinica, 2022, 31(10): 122-134.
| 引物名称Primer name | 引物序列 Primer sequence (5'-3') | 用途Usage |
|---|---|---|
| Gm4CL2-F | GCTCTAGAATGATAACTCTAGC | 基因克隆与表达载体构建 Gene cloning and expression vector construction |
| Gm4CL2-R | TCCTTCTCTTG | |
| qRTGm4CL2-F | TCCCCCGGGAGGCGTCTGAGT | Gm4CL2表达荧光定量 Fluorescence quantitation of Gm4CL2 expression |
| qRTGm4CL2-R | GGCGGCGGTTTC | |
| q18S rRNA-F | CCTCCCTCTCCACTCCTACTG | 内参基因 Internal reference gene |
| q18S rRNA-R | GGAAATGAGGTGGGTGTCGGC | |
| qAtMATE-F | GCATAGGACTTCCGTTTGTGGCA | AtMATE表达荧光定量 Fluorescence quantitation of AtMATE expression |
| qAtMATE-R | CGAACACAAACGCTAAGGCA | |
| qAtSTAR1-F | ACTGTTGCGGATAATGTGAGATA | AtSTAR1表达荧光定量 Fluorescence quantitation of AtSTAR1 expression |
| qAtSTAR1-R | AGAGCACTTGTTGGTTCATCGA | |
| QAtSTAR2-F | AGAAGACGACGACAAAACAAAAA | AtSTAR2表达荧光定量 Fluorescence quantitation of AtSTAR2 expression |
| QAtSTAR2-R | ATGAACTGAAGAACAAATCCGA | |
| qAtactin-F | GGCTCCTCTTAACCCAAAGGC | 内参基因 Internal reference gene |
| qAtactin-R | CACACCATCACCAGAATCCAG |
表1 引物序列信息
Table 1 Primer information
| 引物名称Primer name | 引物序列 Primer sequence (5'-3') | 用途Usage |
|---|---|---|
| Gm4CL2-F | GCTCTAGAATGATAACTCTAGC | 基因克隆与表达载体构建 Gene cloning and expression vector construction |
| Gm4CL2-R | TCCTTCTCTTG | |
| qRTGm4CL2-F | TCCCCCGGGAGGCGTCTGAGT | Gm4CL2表达荧光定量 Fluorescence quantitation of Gm4CL2 expression |
| qRTGm4CL2-R | GGCGGCGGTTTC | |
| q18S rRNA-F | CCTCCCTCTCCACTCCTACTG | 内参基因 Internal reference gene |
| q18S rRNA-R | GGAAATGAGGTGGGTGTCGGC | |
| qAtMATE-F | GCATAGGACTTCCGTTTGTGGCA | AtMATE表达荧光定量 Fluorescence quantitation of AtMATE expression |
| qAtMATE-R | CGAACACAAACGCTAAGGCA | |
| qAtSTAR1-F | ACTGTTGCGGATAATGTGAGATA | AtSTAR1表达荧光定量 Fluorescence quantitation of AtSTAR1 expression |
| qAtSTAR1-R | AGAGCACTTGTTGGTTCATCGA | |
| QAtSTAR2-F | AGAAGACGACGACAAAACAAAAA | AtSTAR2表达荧光定量 Fluorescence quantitation of AtSTAR2 expression |
| QAtSTAR2-R | ATGAACTGAAGAACAAATCCGA | |
| qAtactin-F | GGCTCCTCTTAACCCAAAGGC | 内参基因 Internal reference gene |
| qAtactin-R | CACACCATCACCAGAATCCAG |
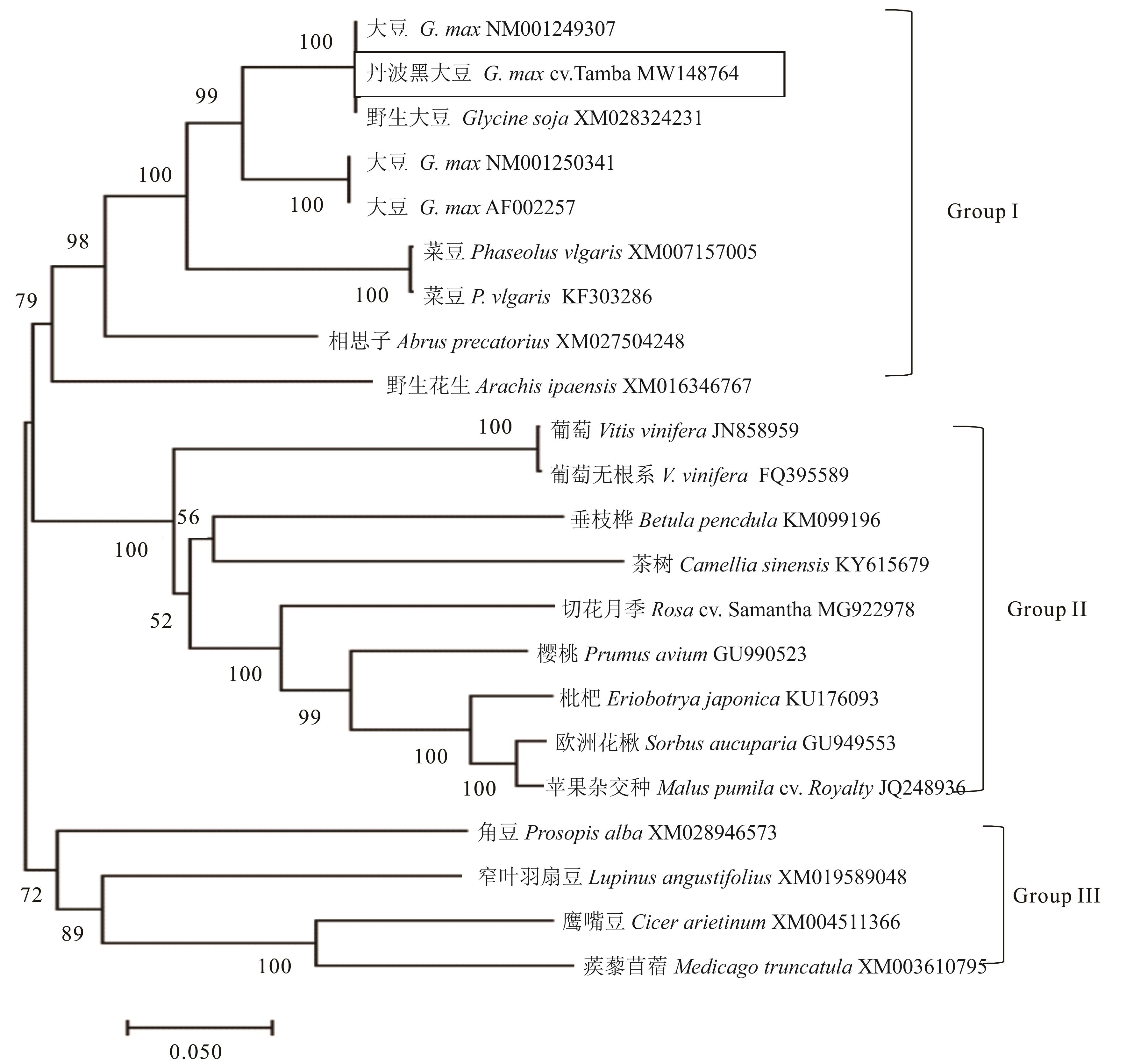
图1 双子叶植物Gm4CL2蛋白的进化分析方框标记为丹波黑大豆Gm4CL2蛋白。The Gm4CL2 protein of Tamba black soybean is marked in box.
Fig. 1 Phylogenetic tree of Gm4CL2 protein in dicotyledons
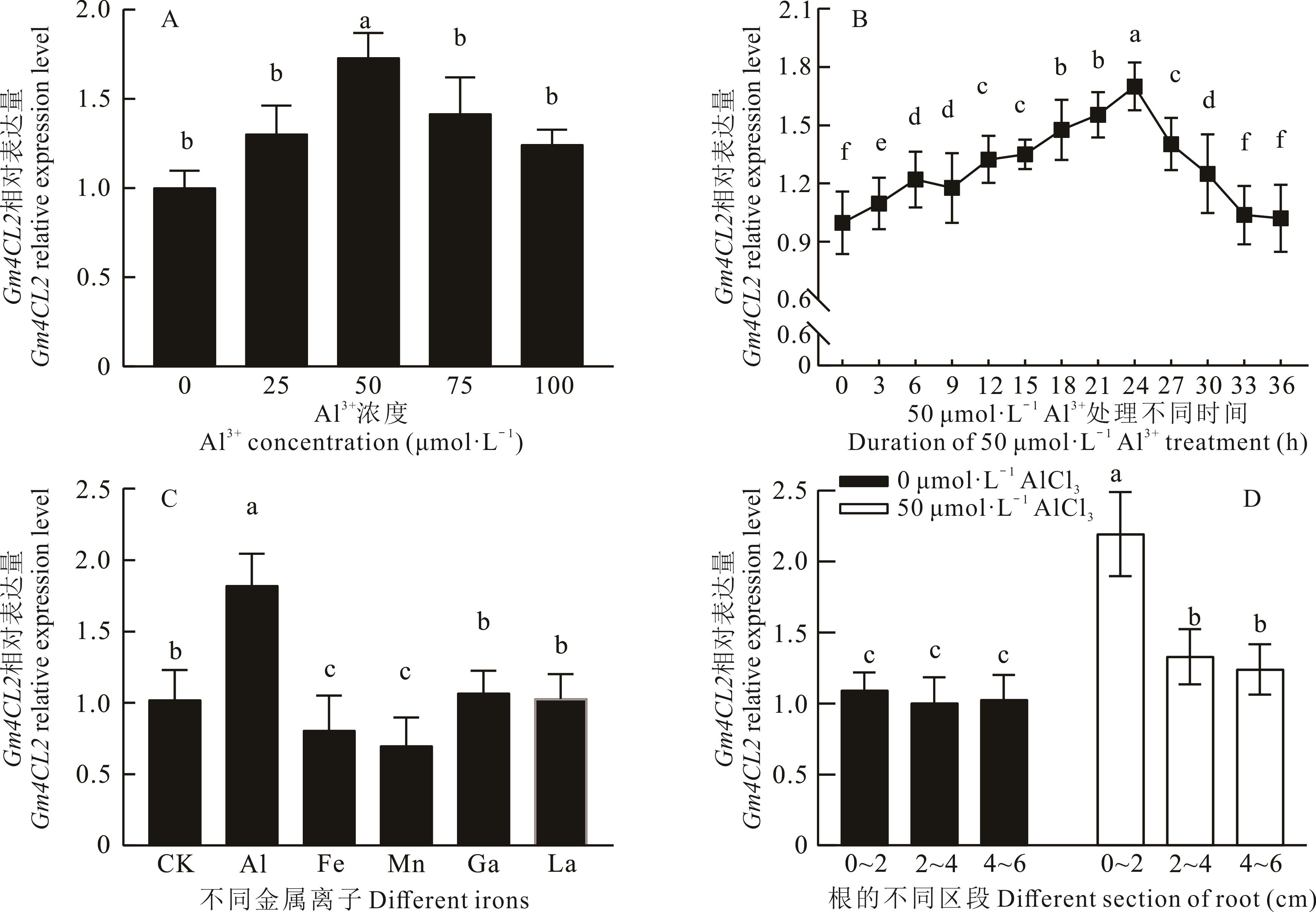
图2 AlCl3胁迫下丹波黑大豆根尖Gm4CL2相对于β-actin的表达量(平均值±标准偏差)A:不同浓度AlCl3处理24 h后,0~2 cm根尖Gm4CL2表达量;B:50 μmol·L-1 AlCl3处理不同时间后,0~2 cm根尖Gm4CL2表达量;C:分别在50 μmol·L-1 AlCl3 (Al)、FeCl3 (Fe)、MnCl3 (Mn)、GaCl3 (Ga)和LaCl3 (La)处理24 h后,0~2 cm根尖Gm4CL2表达量,CK:未处理对照;D: 50 μmol·L-1 AlCl3 处理24 h后,根不同区段Gm4CL2表达量。柱形图上相同字母表示不存在显著性差异(P>0.05),不同字母表示存在显著性差异(P<0.05)。下同。A: Expression levels of Gm4CL2 in 0-2 cm root tips treated with AlCl3 at different concentrations for 24 h; B: Expression levels of Gm4CL2 in 0-2 cm root tips treated with 50 μmol·L-1 AlCl3 during different treatment time; C: Under treatment of AlCl3 (Al)、FeCl3 (Fe)、MnCl3 (Mn)、GaCl3 (Ga)和LaCl3 (La) at 50 μmol·L-1 for 24 h, respectively, expression levels of Gm4CL2 in 0-2 cm root tips. CK: Untreatment control; D: Expression levels of Gm4CL2 in different root segments treated with 50 μmol·L-1 AlCl3 for 24 h. The same letters above columns indicate no significant difference (P>0.05), while different letters indicate significant difference (P<0.05). The same below.
Fig.2 Expression levels of Gm4CL2 related to β-actin (Mean±SD, n=3)

图3 过表达Gm4CL2对Al3+胁迫下拟南芥根系伸长量的影响(平均值±标准偏差)A: Gm4CL2蛋白在拟南芥根尖亚细胞定位;B、C: Gm4CL2过表达和野生型拟南芥在不同浓度AlCl3胁迫24 h根伸长的差异。eGFP: 过表达增强绿色荧光蛋白;4CL2-eGFP: 过表达Gm4CL2和eGFP蛋白。WT: 野生型;4CL2-3、4CL2-5、4CL2-11: 过表达Gm4CL2基因的植株,编号分别为3、5和11。下同。A: Subcellular localization of Gm4CL2 protein in Arabidopsis root tips; B and C: Differences in root elongation between transgenic and wild-type Arabidopsis under different concentrations of AlCl3 stress. eGFP: Overexpression of enhanced green fluorescent protein; 4CL2-eGFP: Overexpression of Gm4CL2 and eGFP proteins. WT: Wild-type; 4CL2-3, 4CL2-5 and 4CL2-11: The plants overexpressed Gm4CL2 gene and were numbered 3, 5 and 11, respectively. The same below.
Fig.3 Effect of Gm4CL2 overexpression on Arabidopsis root elongation under Al3+ stress (Mean±SD, n=6)
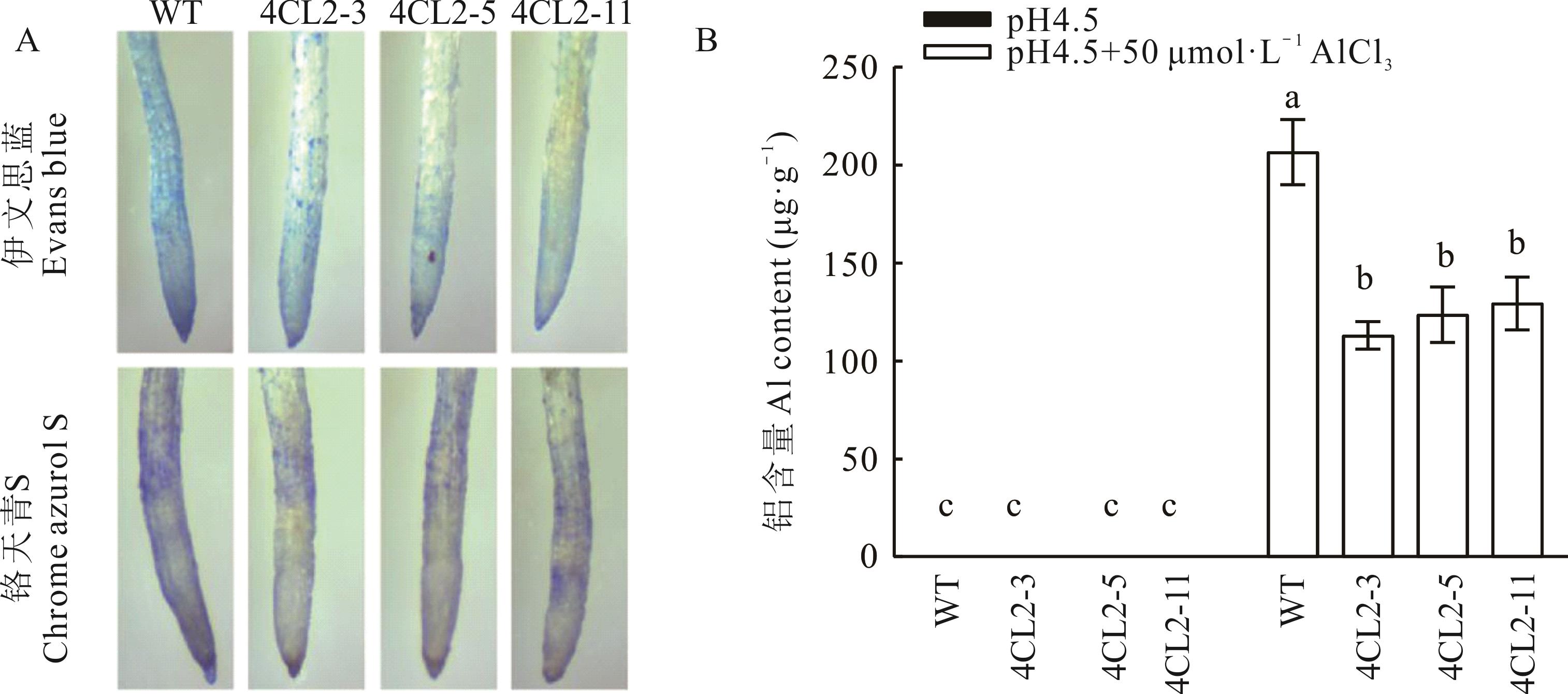
图4 Al3+胁迫24 h Gm4CL2过表达拟南芥根部染色(A)和Al3+含量(B)检测(平均值±标准偏差)
Fig.4 Root staining (A) and Al3+ content (B) of Gm4CL2 overexpression Arabidopsis under Al3+ stress for 24 h (Mean±SD, n=5)
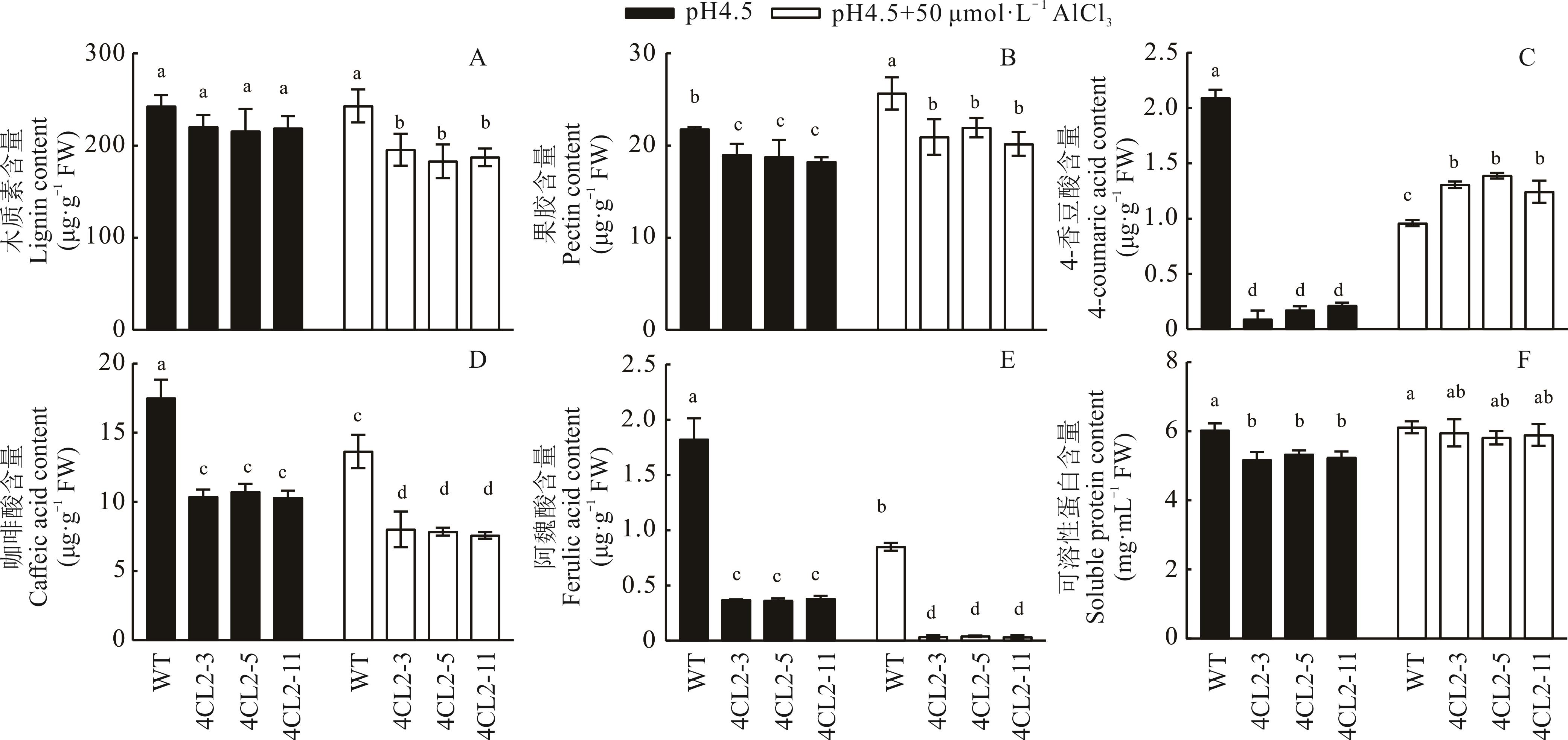
图6 Gm4CL2过表达拟南芥根部细胞壁成分和4CL代谢底物检测(平均值±标准偏差)
Fig.6 Cell wall component and 4CL metabolic substrate analysis in Gm4CL2 overexpression Arabidopsis (Mean±SD, n=3)
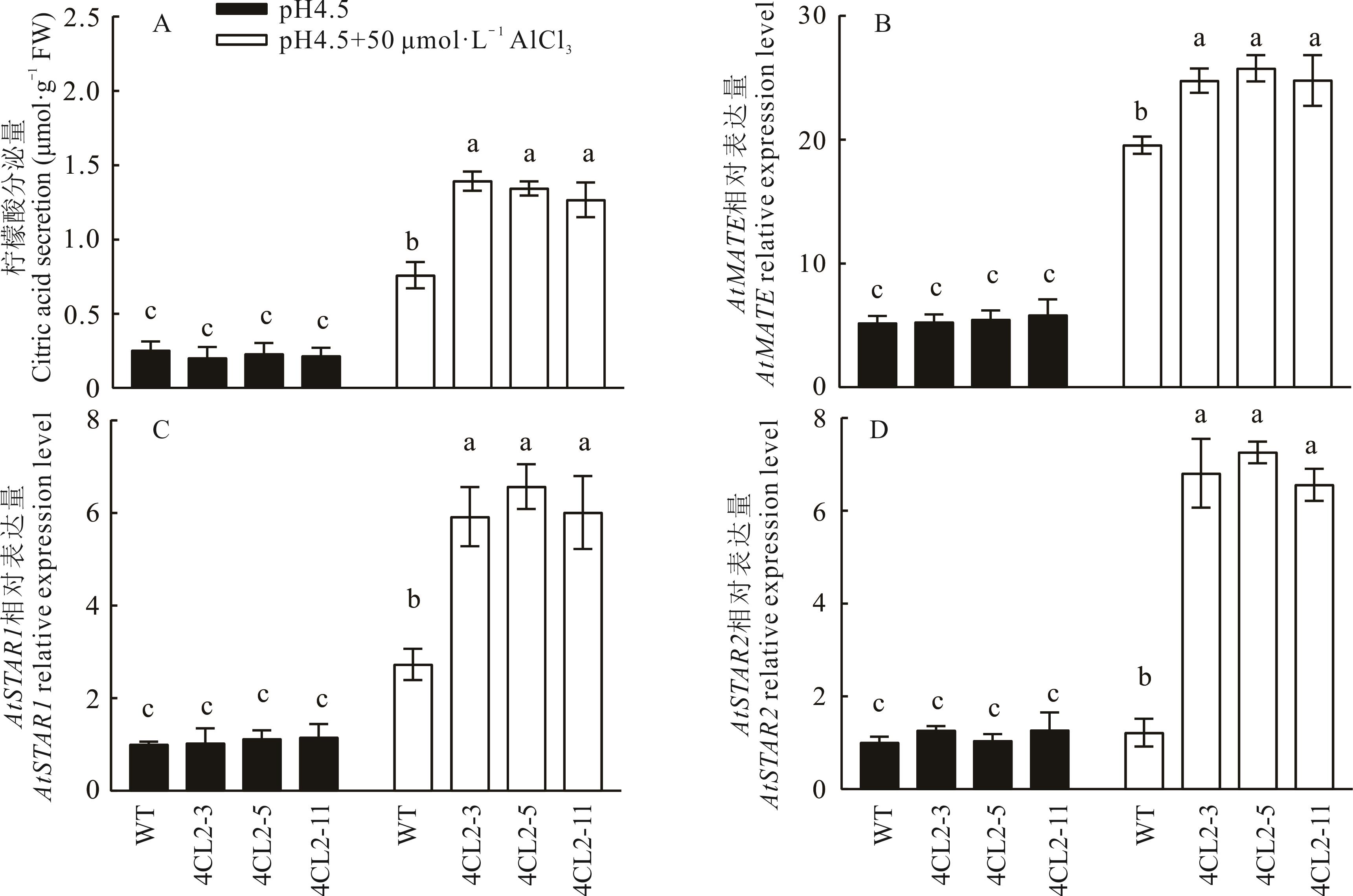
图7 Gm4CL2对拟南芥根尖柠檬酸分泌以及AtMATE、AtSTAR1和AtSTAR2表达的影响(平均值±标准偏差)
Fig.7 Effect of Gm4CL2 on citrate secretion as well as AtMATE, AtSTAR1 and AtSTAR2 expression in Arabidopsis (Mean±SD, n=3)

图8 Gm4CL2过表达紫花苜蓿耐铝性和生物量检测(平均值±标准偏差)A:Gm4CL2过表达和野生型紫花苜蓿Gm4CL2表达水平;B~G:在15 μmol·L-1 AlCl3 (pH 4.5)处理下,Gm4CL2过表达和野生型紫花苜蓿相对未胁迫根伸长率、柠檬酸分泌量、伊文思蓝染色、Al3+含量、木质素含量和生物量。4CL2-1、4CL2-2和4CL2-3:过表达Gm4CL2基因的植株,分别编号为1、2和3。A: Gm4CL2 expression levels in Gm4CL2 overexpression and wild-type alfalfa; B-G: Under 15 μmol·L-1 AlCl3 (pH 4.5) stress, root relative elongation to the untreated control, citric acid secretion, evans blue staining, root tip Al3+ content, root tip lignin content and biomass of Gm4CL2 overexpression and wild-type alfalfa, respectively. 4CL2-1, 4CL2-2 and 4CL2-3: The plants over-expressed Gm4CL2 gene and were numbered 1, 2 and 3, respectively.
Fig. 8 Determination of aluminum tolerance and biomass in Gm4CL2 overexpression alfalfa (Mean±SD, n=3)
| 1 | Yang J L, Fan W, Zheng S J. Mechanisms and regulation of aluminum-induced secretion of organic acid anions from plant roots. Journal of Zhejiang University-Science B, 2019, 20(6): 513-527. |
| 2 | Zhang X, Long Y, Huang J, et al. Molecular mechanisms for coping with Al toxicity in plants. International Journal of Molecular Sciences, 2019, 20(7): 1551. |
| 3 | Hanson A A, Barnes D K, Hill R R. Alfalfa and alfalfa improvement. Madison, Wisconsin, USA: American Society of Agronomy, Inc., 1988. |
| 4 | Ryan P R, Dong D, Teuber F, et al. Assessing how the aluminum-resistance traits in wheat and rye transfer to hexaploid and octoploid triticale. Frontiers in Plant Science, 2018, 9: 1334. |
| 5 | Lavhale S G, Kalunke R M, Giri A P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta, 2018, 248(5): 1063-1078. |
| 6 | Zhang C H, Ma T, Luo W C, et al. Identification of 4CL genes in desert poplars and their changes in expression in response to salt stress. Genes, 2015, 6: 901-917. |
| 7 | Naik P, Wang J P, Sederof R, et al. Assessing the impact of the 4CL enzyme complex on the robustness of monolignol biosynthesis using metabolic pathway analysis. PLoS One, 2018, 13(3): e0193896. |
| 8 | Wei Y, Jiang C, Han R, et al. Plasma membrane proteomic analysis by TMT-PRM provides insight into mechanisms of aluminum resistance in tamba black soybean roots tips. PeerJ, 2020, 8: e9312. |
| 9 | Wang J, Gao X, Dong J, et al. Over-expression of the heat-responsive wheat gene TaHSP23.9 in transgenic Arabidopsis conferred tolerance to heat and salt stress. Frontiers in Plant Science, 2020(11): 266-270. |
| 10 | Gan Z C, Chen D Y, Zhang L, et al. Research on aluminum tolerance of citrate synthase transgenic alfalfa. Scientia Agricultura Sinica, 2010, 43(16): 3461-3466. |
| 甘智才, 陈东颖, 张丽, 等. 转柠檬酸合成酶基因苜蓿耐铝性研究. 中国农业科学, 2010, 43(16): 3461-3466. | |
| 11 | Wei Y M. Plasma membrane proteomic analysis provides insight into mechanisms of aluminum resistance and functional haracterization and utilization of related differentially expressed proteins in Tamba black soybean. Chongqing: Southwest University, 2020. |
| 魏运民. 基于质膜蛋白组学探究丹波黑大豆耐铝的分子机制及相关差异蛋白的功能鉴定与利用. 重庆: 西南大学, 2020. | |
| 12 | Jiang C, Liu L, Li X, et al. Insights into aluminum-tolerance pathways in Stylosanthes as revealed by RNA-seq analysis. Scientific Reports, 2018, 8(1): 6072. |
| 13 | Liu S, Zhao L, Liao Y, et al. Dysfunction of the 4-coumarate: coenzyme A ligase 4CL4 impacts aluminum resistance and lignin accumulation in rice. Plant Journal, 2020, 104(5): 1233-1250. |
| 14 | Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. Journal of Experimental Botany, 2014, 65(5): 1229-1240. |
| 15 | Chen X H, Su W L, Zhang H, et al. Fraxinus mandshurica 4-coumarate-CoA ligase 2 enhances drought and osmotic stress tolerance of tobacco by increasing coniferyl alcohol content. Plant Physiology and Biochemistry, 2020, 155: 697-708. |
| 16 | Sun S C, Xiong X P, Zhang X L, et al. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance. BMC Plant Biology, 2020, 20: 125. |
| 17 | Wohl J, Petersen M. Phenolic metabolism in the hornwort Anthoceros agrestis: 4-coumarate CoA ligase and 4-hydroxybenzoate CoA ligase. Plant Cell Reports, 2020, 39: 1129-1141. |
| 18 | Mohammad A, Hossain S B. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Frontiers in Plant Science, 2015, 6: 420. |
| 19 | Jain G, Gould K S. Are betalain pigments the functional homologues of anthocyanins in plants. Environmental and Experimental Botany, 2015, 119: 48-53. |
| 20 | Ullah A, Sun H, Yan X, et al. A novel cotton WRKY-gene, GhWRKY6-like, improves salt tolerance by activating the ABA signalling pathway and scavenging of reactive oxygen species. Physiologia Plantarum, 2018, 162(4): 439-454. |
| 21 | Chen X, Wang H, Li X, et al. Molecular cloning and functional analysis of 4-Coumarate: CoA ligase 4 (4CL-like 1) from Fraxinus mandshurica and its role in abiotic stress tolerance and cell wall synthesis. BMC Plant Biology, 2019, 19(1): 231. |
| 22 | Tian X M, Yan L H, Xiang G F, et al. Research progress on 4-coumarate: coenzyme A ligase (4CL) in plants. Biotechnology Bulletin, 2017, 33(4): 19-26. |
| 田晓明, 颜立红, 向光锋, 等. 植物4-香豆酸: 辅酶A连接酶研究进展. 生物技术通报, 2017, 33(4): 19-26. | |
| 23 | Hu J, Qi Q, Zhao Y, et al. Unraveling the impact of Pto4CL1 regulation on the cell wall components and wood properties of perennial transgenic Populus tomentosa. Plant Physiology and Biochemistry, 2019, 139: 672-680. |
| 24 | Ma Q, Yi R, Li L, et al. GsMATE encoding a multidrug and toxic compound extrusion transporter enhances aluminum tolerance in Arabidopsis thaliana. BMC Plant Biology, 2018, 18(1): 1-10. |
| 25 | Zuo F H, Ling G Z, Tang X L, et al. Al stress-induced citrate secretion from roots in Stylosanthes. Scientia Agricultura Sinica, 2010, 43(1): 59-64. |
| 左方华, 凌桂芝, 唐新莲, 等. 铝胁迫诱导柱花草根系分泌柠檬酸. 中国农业科学, 2010, 43(1): 59-64. | |
| 26 | Ma Q, Yan Q, Zhang Z S, et al. Identification, evolution and expression analysis of the CCoAOMT family genes in Medicago sativa. Acta Prataculturae Sinica, 2021, 30(11): 144-156. |
| 马倩, 闫启, 张正社, 等. 紫花苜蓿CCoAOMT基因家族的鉴定、进化及表达分析. 草业学报, 2021, 30(11): 144-156. |
| [1] | 孙延亮, 赵俊威, 刘选帅, 李生仪, 马春晖, 王旭哲, 张前兵. 施氮对苜蓿初花期光合日变化、叶片形态及干物质产量的影响[J]. 草业学报, 2022, 31(9): 63-75. |
| [2] | 王星, 黄薇, 余淑艳, 李小云, 高雪芹, 伏兵哲. 宁夏地区地下滴灌水肥耦合对紫花苜蓿种子产量及构成因素的影响[J]. 草业学报, 2022, 31(9): 76-85. |
| [3] | 赵建涛, 岳亚飞, 张前兵, 马春晖. 不同秋眠级紫花苜蓿品种抗寒性对新疆北疆地区覆雪厚度的响应[J]. 草业学报, 2022, 31(8): 24-34. |
| [4] | 刘彩婷, 毛丽萍, 阿依谢木, 于应文, 沈禹颖. 紫花苜蓿与垂穗披碱草混播比例对其抗寒生长生理特征的影响[J]. 草业学报, 2022, 31(7): 133-143. |
| [5] | 王雪萌, 何欣, 张涵, 宋瑞, 毛培胜, 贾善刚. 基于多光谱成像技术快速无损检测紫花苜蓿人工老化种子[J]. 草业学报, 2022, 31(7): 197-208. |
| [6] | 张晴, 邢静, 姚佳明, 殷庭超, 黄心如, 何悦, 张敬, 徐彬. 多年生黑麦草细胞分裂素信号通路B类ARR转录因子LpARR10的耐镉功能分析[J]. 草业学报, 2022, 31(5): 135-143. |
| [7] | 李满有, 李东宁, 王斌, 李小云, 沈笑天, 曹立娟, 倪旺, 王腾飞, 兰剑. 不同苜蓿品种混播和播种量对牧草产量及品质的影响[J]. 草业学报, 2022, 31(5): 61-75. |
| [8] | 孙洪仁, 王显国, 卜耀军, 乔楠, 任波. 黄土高原紫花苜蓿土壤氮素丰缺指标和推荐施氮量初步研究[J]. 草业学报, 2022, 31(4): 32-42. |
| [9] | 高丽敏, 陈春, 沈益新. 氮磷肥对季节性栽培紫花苜蓿生长及再生的影响[J]. 草业学报, 2022, 31(4): 43-52. |
| [10] | 欧成明, 赵美琦, 孙铭, 毛培胜. 抗坏血酸和水杨酸丸衣对NaCl胁迫下紫花苜蓿种子发芽特性的影响[J]. 草业学报, 2022, 31(4): 93-101. |
| [11] | 童长春, 刘晓静, 吴勇, 赵雅姣, 王静. 内源异黄酮对紫花苜蓿结瘤固氮及氮效率的调控研究[J]. 草业学报, 2022, 31(3): 124-135. |
| [12] | 张岳阳, 李芳, 梁维维, 李彦忠. 新疆昌吉32个紫花苜蓿品种的田间抗病性评价[J]. 草业学报, 2022, 31(2): 133-146. |
| [13] | 王斌, 杨雨琦, 李满有, 倪旺, 海艺蕊, 张顺香, 董秀, 兰剑. 不同播种量下行距配置对紫花苜蓿产量及品质的影响[J]. 草业学报, 2022, 31(2): 147-158. |
| [14] | 张辉辉, 师尚礼, 武蓓, 李自立, 李小龙. 苜蓿与3种多年生禾草混播效应研究[J]. 草业学报, 2022, 31(2): 159-170. |
| [15] | 白婕, 臧真凤, 刘丛, 昝看卓, 龙明秀, 王可珍, 屈洋, 何树斌. 紫花苜蓿叶片和根系膜脂过氧化及C、N特征对水分和N添加的响应[J]. 草业学报, 2022, 31(2): 213-220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||