

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2024, Vol. 33 ›› Issue (5): 115-127.DOI: 10.11686/cyxb2023239
• 研究论文 • 上一篇
收稿日期:2023-07-12
修回日期:2023-09-19
出版日期:2024-05-20
发布日期:2024-02-03
通讯作者:
代金霞
作者简介:E-mail: daijx05@163.com基金资助:
Shuang LIU( ), Jia-ni YAO, Jun-jie ZHANG, Jin-xia DAI(
), Jia-ni YAO, Jun-jie ZHANG, Jin-xia DAI( )
)
Received:2023-07-12
Revised:2023-09-19
Online:2024-05-20
Published:2024-02-03
Contact:
Jin-xia DAI
摘要:
为探索宁夏荒漠草原豆科灌丛根际土壤中参与氨氧化和反硝化的微生物功能基因丰度和群落组成对植物类型和生长时期的响应特征,本研究以宁夏白芨滩国家级自然保护区内典型的豆科灌丛群落柠条、沙冬青、猫头刺和毛刺不同生长时期(营养期、盛花期和果实期)的根际土壤为材料,通过功能基因实时荧光定量PCR和高通量测序方法,分析4种灌丛根际土壤中氨氧化和反硝化微生物功能基因丰度、群落结构组成特征及其与土壤因子的相关性。结果表明,功能基因丰度及群落结构因灌丛类型和生长时期不同而存在差异。柠条灌丛根际土壤中各功能基因丰度显著高于其他3种灌丛,且在盛花期达到最高。柠条各生长期根际土壤中氨氧化细菌(ammonia-oxidizing bacteria, AOB)群落多样性高于其他灌丛类型,而毛刺根际氨氧化古菌(ammonia-oxidizing archaea, AOA)和nirK型反硝化菌群落多样性最低。各灌丛根际AOA以未分类类群为绝对优势,占79.34%~98.37%,奇古菌门和泉古菌门分别仅占0.28%~20.37%和0.28%~2.35%;AOB在柠条根际以变形菌门为优势,其他3种植物根际则以未分类类群为优势;nirK和nirS型反硝化菌均以变形菌门为优势门,但nirK型反硝化菌的组成受植物类型和生长时期的影响更显著。功能基因的丰度与土壤全氮、速效钾和pH显著正相关。全钾、速效钾、有机质显著影响氨氧化微生物的群落组成,pH是反硝化微生物群落结构的主要影响因子。
刘爽, 姚佳妮, 张钧杰, 代金霞. 荒漠豆科灌丛根际土壤氨氧化和反硝化微生物功能基因丰度及群落多样性特征[J]. 草业学报, 2024, 33(5): 115-127.
Shuang LIU, Jia-ni YAO, Jun-jie ZHANG, Jin-xia DAI. Functional gene abundance and community diversity of ammonia-oxidizing and denitrifying microorganisms in the rhizosphere soil of desert leguminous shrubs[J]. Acta Prataculturae Sinica, 2024, 33(5): 115-127.
| 功能基因Functional genes | 引物Primers | 引物序列Primer sequence (5′-3′) | 长度Length (bp) | 参考文献Reference |
|---|---|---|---|---|
| amoA | amoAF | STAATGGTCTGGCTTAGACG | 600 | [ |
| amoAR | GCGGCCATCCATCTGTATGT | |||
| bamoA | bamoA1F | GGGGTTTCTACTGGTGGT | 491 | [ |
| bamoA2R | CCCCTCKGSAAAGCCTTCTTC | |||
| nirK | nirK1aCuF | ATCATGGTSCTGCCGCG | 459 | [ |
| nirK3aCuR | GCCTCGATCAGRTTGTGGTT | |||
| nirS | nirS4F | TTCRTCAAGACSCAYCCGAA | 332 | [ |
| nirS6R | CGTTGAACTTRCCGGT |
表1 氨氧化和反硝化功能基因实时荧光定量PCR的引物
Table 1 Primers for real-time fluorescent quantitative PCR of functional genes related to ammonia oxidation and denitrification
| 功能基因Functional genes | 引物Primers | 引物序列Primer sequence (5′-3′) | 长度Length (bp) | 参考文献Reference |
|---|---|---|---|---|
| amoA | amoAF | STAATGGTCTGGCTTAGACG | 600 | [ |
| amoAR | GCGGCCATCCATCTGTATGT | |||
| bamoA | bamoA1F | GGGGTTTCTACTGGTGGT | 491 | [ |
| bamoA2R | CCCCTCKGSAAAGCCTTCTTC | |||
| nirK | nirK1aCuF | ATCATGGTSCTGCCGCG | 459 | [ |
| nirK3aCuR | GCCTCGATCAGRTTGTGGTT | |||
| nirS | nirS4F | TTCRTCAAGACSCAYCCGAA | 332 | [ |
| nirS6R | CGTTGAACTTRCCGGT |

图1 4种豆科灌丛根际土壤中氨氧化和反硝化功能基因丰度不同大写字母表示同一生长时期不同灌丛之间差异显著(P<0.05),不同小写字母表示同一灌丛不同生长时期之间差异显著(P<0.05)。Different capital letters represent significant differences among different shrubs at the same growth period (P<0.05), while different lowercase letters represent significant differences among different growth periods of the same shrub (P<0.05).
Fig.1 Abundance of functional genes related to ammonia oxidation and denitrification in rhizosphere soil of four leguminous shrubs
样品编号 Sample number | 氨氧化古菌 Ammonia-oxidizing archaea | 氨氧化细菌 Ammonia-oxidizing bacteria | nirK型反硝化菌 nirK-type denitrifying bacteria | nirS型反硝化菌 nirS-type denitrifying bacteria | ||||
|---|---|---|---|---|---|---|---|---|
| Chao 1 | Shannon | Chao 1 | Shannon | Chao 1 | Shannon | Chao 1 | Shannon | |
| SDQ1 | 23.00 | 1.4690 | 22.00 | 1.7425 | 274.69 | 3.9031 | 105.60 | 2.0859 |
| SDQ2 | 30.00 | 1.8068 | 23.00 | 1.7253 | 169.50 | 3.4446 | 119.43 | 3.5405 |
| SDQ3 | 27.50 | 1.3555 | 19.00 | 1.8469 | 211.50 | 3.3924 | 92.33 | 2.7693 |
| NT1 | 23.00 | 1.2244 | 35.00 | 2.2478 | 291.13 | 3.3304 | 152.20 | 3.2573 |
| NT2 | 18.00 | 1.2407 | 30.00 | 2.3161 | 271.02 | 3.4343 | 155.24 | 3.1518 |
| NT3 | 24.00 | 1.3009 | 32.00 | 2.4139 | 298.03 | 4.1252 | 114.50 | 3.5996 |
| MTC1 | 28.00 | 1.9160 | 24.00 | 1.6897 | 204.45 | 3.3538 | 113.00 | 2.9958 |
| MTC2 | 24.50 | 1.5782 | 37.00 | 1.6630 | 220.30 | 3.5430 | 136.65 | 3.3694 |
| MTC3 | 24.33 | 1.5728 | 38.00 | 2.0907 | 205.12 | 3.1738 | 68.00 | 2.0021 |
| MC1 | 23.33 | 0.6176 | 10.00 | 1.1828 | 162.00 | 2.5022 | 66.00 | 3.0166 |
| MC2 | 20.00 | 0.7633 | 16.00 | 1.7892 | 177.96 | 3.1210 | 100.43 | 3.0036 |
| MC3 | 23.20 | 0.5309 | 25.50 | 1.7542 | 160.00 | 2.4473 | 82.50 | 3.0530 |
表2 4种豆科灌丛根际土壤氨氧化和反硝化微生物多样性指数
Table 2 Diversity index of ammonia-oxidizing and denitrifying microorganisms in rhizosphere soil of four leguminous shrubs
样品编号 Sample number | 氨氧化古菌 Ammonia-oxidizing archaea | 氨氧化细菌 Ammonia-oxidizing bacteria | nirK型反硝化菌 nirK-type denitrifying bacteria | nirS型反硝化菌 nirS-type denitrifying bacteria | ||||
|---|---|---|---|---|---|---|---|---|
| Chao 1 | Shannon | Chao 1 | Shannon | Chao 1 | Shannon | Chao 1 | Shannon | |
| SDQ1 | 23.00 | 1.4690 | 22.00 | 1.7425 | 274.69 | 3.9031 | 105.60 | 2.0859 |
| SDQ2 | 30.00 | 1.8068 | 23.00 | 1.7253 | 169.50 | 3.4446 | 119.43 | 3.5405 |
| SDQ3 | 27.50 | 1.3555 | 19.00 | 1.8469 | 211.50 | 3.3924 | 92.33 | 2.7693 |
| NT1 | 23.00 | 1.2244 | 35.00 | 2.2478 | 291.13 | 3.3304 | 152.20 | 3.2573 |
| NT2 | 18.00 | 1.2407 | 30.00 | 2.3161 | 271.02 | 3.4343 | 155.24 | 3.1518 |
| NT3 | 24.00 | 1.3009 | 32.00 | 2.4139 | 298.03 | 4.1252 | 114.50 | 3.5996 |
| MTC1 | 28.00 | 1.9160 | 24.00 | 1.6897 | 204.45 | 3.3538 | 113.00 | 2.9958 |
| MTC2 | 24.50 | 1.5782 | 37.00 | 1.6630 | 220.30 | 3.5430 | 136.65 | 3.3694 |
| MTC3 | 24.33 | 1.5728 | 38.00 | 2.0907 | 205.12 | 3.1738 | 68.00 | 2.0021 |
| MC1 | 23.33 | 0.6176 | 10.00 | 1.1828 | 162.00 | 2.5022 | 66.00 | 3.0166 |
| MC2 | 20.00 | 0.7633 | 16.00 | 1.7892 | 177.96 | 3.1210 | 100.43 | 3.0036 |
| MC3 | 23.20 | 0.5309 | 25.50 | 1.7542 | 160.00 | 2.4473 | 82.50 | 3.0530 |

图2 4种豆科灌丛根际土壤氨氧化微生物在门和属水平的群落组成AOA: 氨氧化古菌Ammonia-oxidizing archaea; AOB: 氨氧化细菌Ammonia-oxidizing bacteria. SDQ: 沙冬青A. mongolicus; NT: 柠条C. korshinskii; MTC: 猫头刺O. aciphylla; MC: 毛刺C. tibetica. 1、2、3分别代表营养期、盛花期和果实期。1, 2, and 3 respectively represent the nutritional period, flowering period, and fruit period. 下同The same below.
Fig.2 Community composition of ammonia-oxidizing microorganisms in rhizosphere soil of four leguminous shrubs at the phylum and genus levels
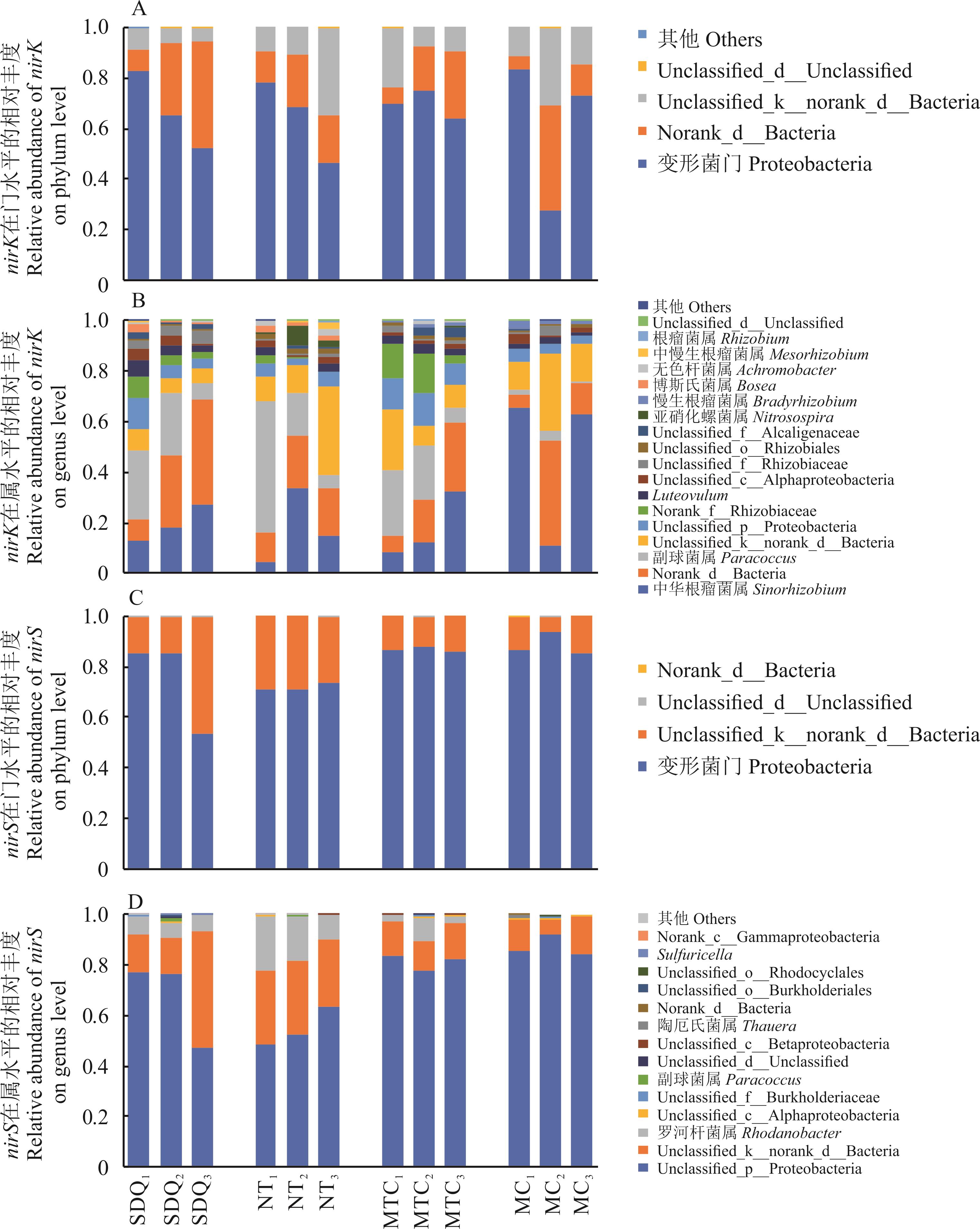
图3 4种豆科灌丛根际土壤反硝化微生物在门和属水平的群落组成
Fig.3 Community composition of denitrifying microorganisms in rhizosphere soil of four leguminous shrubs at phylum and genus levels
基因 Gene | 全氮 Total N | 全磷 Total P | 全钾 Total K | 有机质 Soil organic matter (SOM) | 硝态氮 Nitrate N | 铵态氮 Ammonium N | 亚硝酸盐氮Nitrite N | 速效磷 Available P | 速效钾 Available K | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| amoA | 0.690* | 0.600* | 0.724** | 0.839** | 0.207 | 0.183 | 0.183 | 0.483 | 0.798** | 0.685* |
| bamoA | 0.784** | 0.689* | 0.656* | 0.789** | 0.451 | 0.370 | 0.300 | 0.697* | 0.861** | 0.707* |
| nirK | 0.663* | 0.531 | 0.540 | 0.713** | 0.302 | 0.234 | 0.051 | 0.570 | 0.791** | 0.712** |
| nirS | 0.587* | 0.448 | 0.492 | 0.540 | 0.456 | 0.262 | 0.162 | 0.618* | 0.781** | 0.850** |
表3 氨氧化和反硝化功能基因丰度与土壤理化性质的Pearson相关性分析
Table 3 Pearson correlation analysis between abundance of functional genes related to ammonia oxidation and denitrification and soil physicochemical properties
基因 Gene | 全氮 Total N | 全磷 Total P | 全钾 Total K | 有机质 Soil organic matter (SOM) | 硝态氮 Nitrate N | 铵态氮 Ammonium N | 亚硝酸盐氮Nitrite N | 速效磷 Available P | 速效钾 Available K | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| amoA | 0.690* | 0.600* | 0.724** | 0.839** | 0.207 | 0.183 | 0.183 | 0.483 | 0.798** | 0.685* |
| bamoA | 0.784** | 0.689* | 0.656* | 0.789** | 0.451 | 0.370 | 0.300 | 0.697* | 0.861** | 0.707* |
| nirK | 0.663* | 0.531 | 0.540 | 0.713** | 0.302 | 0.234 | 0.051 | 0.570 | 0.791** | 0.712** |
| nirS | 0.587* | 0.448 | 0.492 | 0.540 | 0.456 | 0.262 | 0.162 | 0.618* | 0.781** | 0.850** |
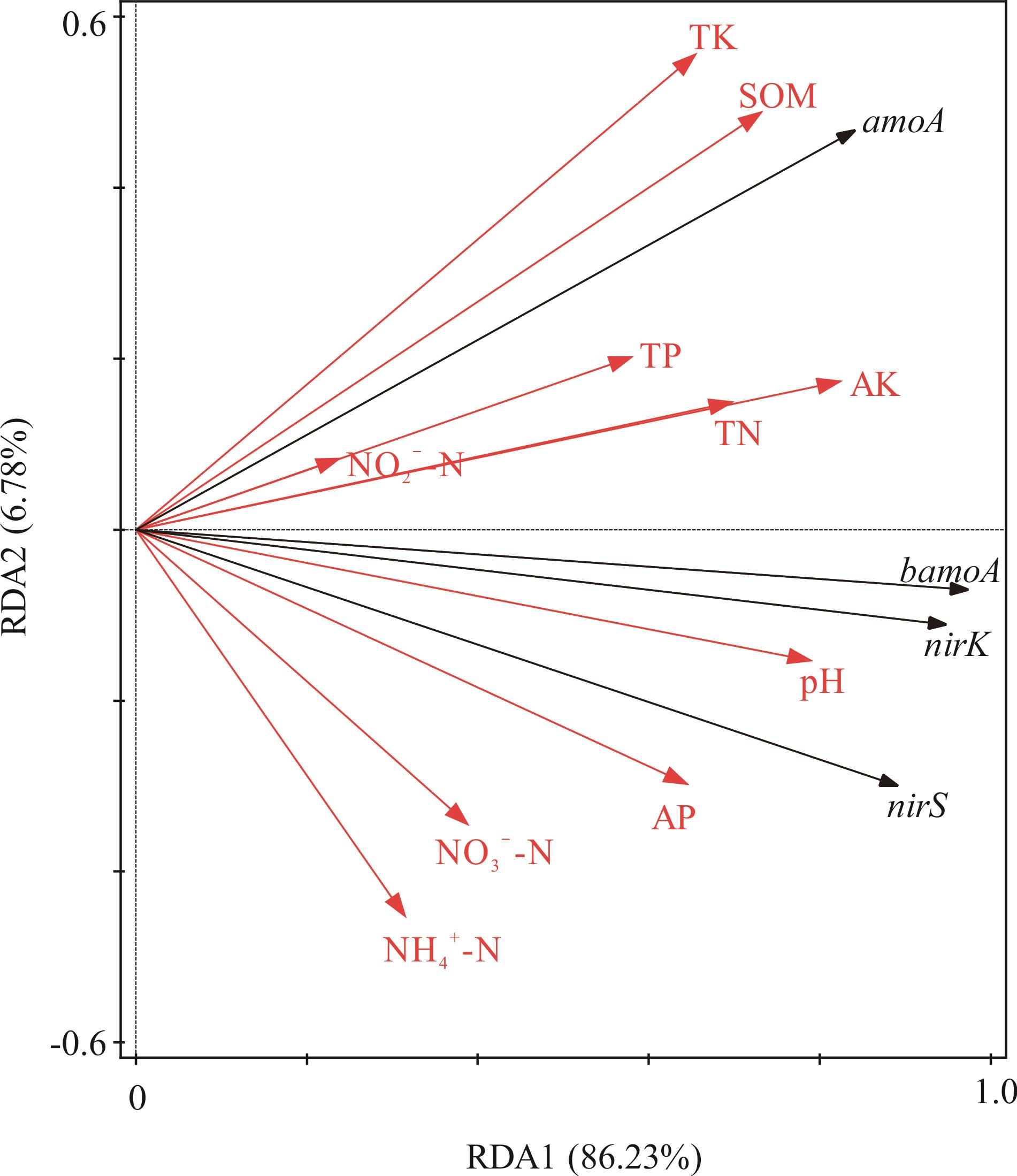
图4 氨氧化和反硝化功能基因丰度与土壤理化性质的冗余分析TN: 全氮Total nitrogen; TP: 全磷Total phosphorous; TK: 全钾Total potassium; SOM: 有机质Soil organic matter; NO3--N: 硝态氮Nitrate nitrogen; NH4+-N: 铵态氮Ammonium nitrogen; NO2--N: 亚硝酸盐氮Nitrite nitrogen; AP: 速效磷Available phosphorous; AK: 速效钾Available potassium. 下同The same below.
Fig.4 Redundancy analysis between abundance of functional genes related to ammonia oxidation and denitrification with soil physicochemical properties

图5 氨氧化和反硝化功能微生物群落与土壤理化因子关联分析A: 氨氧化古菌Ammonia-oxidizing archaea; B: 氨氧化细菌Ammonia-oxidizing bacteria; C: nirK型反硝化菌nirK-type denitrifying bacteria; D: nirS型反硝化菌nirS-type denitrifying bacteria. *: P<0.05; **: P<0.01; ***: P<0.001.
Fig.5 Correlation analysis between ammonia-oxidizing and denitrifying microbial communities with soil physicochemical properties
| 1 | Li T, Zhang W, Liu G X, et al. Advances in the study of microbial ecology in desert soil. Journal of Desert Research, 2018, 38(2): 329-338. |
| 李婷, 张威, 刘光琇, 等. 荒漠土壤微生物群落结构特征研究进展. 中国沙漠, 2018, 38(2): 329-338. | |
| 2 | Naylor D, Coleman-Derr D. Drought stress and root-associated bacterial communities. Frontiers in Plant Science, 2018, 8: 2223. |
| 3 | Levy-Booth D J, Prescott C E, Grayston S J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biology and Biochemistry, 2014, 75: 11-25. |
| 4 | Ouyang Y, Evans S E, Friesen M L, et al. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies. Soil Biology and Biochemistry, 2018, 127: 71-78. |
| 5 | Zhang L. Effects of plant species on soil denitrification in the lakeshore zone of Yezhi Lake. Wuhan: Huazhong Agricultural University, 2022. |
| 张翎. 植物种类对野芷湖湖岸带土壤反硝化作用的影响. 武汉: 华中农业大学, 2022. | |
| 6 | Huhe, Borjigin S, Buhebaoyin, et al. Microbial nitrogen-cycle gene abundance in soil of cropland abandoned for different periods. PLoS One, 2016, 11(5): e0154697. |
| 7 | Zhang B H, Jin D S, Zhang Q, et al. Effects of different plant cultivations on the microbiological diversity of recultivated soil in mining area. Journal of Agricultural Resources and Environment, 2019, 36(3): 355-360. |
| 张变华, 靳东升, 张强, 等. 不同植物种植对矿区复垦土壤微生物多样性的影响. 农业资源与环境学报, 2019, 36(3): 355-360. | |
| 8 | Pan K L, Gao J F, Li H Y, et al. Ammonia-oxidizing bacteria dominate ammonia oxidation in a full-scale wastewater treatment plant revealed by DNA-based stable isotope probing. Bioresource Technology, 2018, 256: 152-159. |
| 9 | Wang X D. Comprehensive scientific investigation report in Baijitan Nature Reserve in Lingwu, Ningxia. Beijing: China Forestry Publishing House, 2018. |
| 王兴东. 宁夏灵武白芨滩自然保护区综合科学考察报告. 北京: 中国林业出版社, 2018. | |
| 10 | Liu S, Yao J N, Shen C, et al. Fluorescent quantitative PCR of nifH gene and diversity analysis of nitrogen-fixing bacteria in the rhizosphere soil of Caragana spp. of desert grassland. Biotechnology Bulletin, 2022, 38(12): 252-262. |
| 刘爽, 姚佳妮, 沈聪, 等. 荒漠植物柠条根际土壤nifH基因荧光定量及固氮菌多样性分析. 生物技术通报, 2022, 38(12): 252-262. | |
| 11 | Lu R K. Analytical methods for soil and agro-chemistry. Beijing: China Agricultural Science and Technology Press, 2000. |
| 鲁如坤. 土壤农业化学分析方法. 北京: 中国农业科技出版社, 2000. | |
| 12 | Zhang M, Liu J J, Liu Z X, et al. Distribution characteristics of microbial gene abundance in key processes of soil nitrogen cycling in black soil zone. Acta Pedologica Sinica, 2022, 59(5): 1258-1269. |
| 张淼, 刘俊杰, 刘株秀, 等. 黑土区农田土壤氮循环关键过程微生物基因丰度的分布特征. 土壤学报, 2022, 59(5): 1258-1269. | |
| 13 | Ren L L. Characteristics of nitrogen-cycling-related functional genes under long-term fertilization in brown earth. Shenyang: Shenyang Agricultural University, 2019. |
| 任灵玲. 长期施肥棕壤中氮代谢功能基因的变化特征. 沈阳: 沈阳农业大学, 2019. | |
| 14 | Mao Y J, Yannarell A C, Mackie R I. Changes in N-transforming archaea and bacteria in soil during the establishment of bioenergy crops. PLoS One, 2011, 6(9): e24750. |
| 15 | Palmer K, Biasi C, Horn M A. Contrasting denitrifier communities relate to contrasting N2O emission patterns from acidic peat soils in arctic tundra. The ISME Journal, 2012, 6(5): 1058-1077. |
| 16 | Liu L, Shen G Q, Sun M X, et al. Effect of biochar on nitrous oxide emission and its potential mechanisms. Journal of the Air & Waste Management Association, 2014, 64(8): 894-902. |
| 17 | Guo H J, Ma L J, Liang Y C, et al. Response of ammonia-oxidizing bacteria and archaea to long-term saline water irrigation in alluvial grey desert soils. Scientific Reports, 2020, 10(1): 489. |
| 18 | Magalhães C M, Machado A, Frank-Fahle B, et al. The ecological dichotomy of ammonia-oxidizing archaea and bacteria in the hyper-arid soils of the Antarctic Dry Valleys. Frontiers in Microbiology, 2014, 5: 515. |
| 19 | Zeng T T, Li D, Xie S B, et al. A review on microbial properties of anaerobic ammonium oxidation (ANAMMOX) bacteria. Chinese Journal of Applied and Environmental Biology, 2014, 20(6): 1111-1116. |
| 曾涛涛, 李冬, 谢水波, 等. 厌氧氨氧化菌微生物特性研究进展. 应用与环境生物学报, 2014, 20(6): 1111-1116. | |
| 20 | Jones C M, Bla S, Magnus R, et al. Phylogenetic analysis of nitrite, nitric oxide, and nitrous oxide respiratory enzymes reveal a complex evolutionary history for denitrification. Molecular Biology & Evolution, 2008, 25(9): 1955-1966. |
| 21 | Desnues C, Michotey V D, Wieland A, et al. Seasonal and diel distributions of denitrifying and bacterial communities in a hypersaline microbial mat (Camargue, France). Water Research, 2007, 41(15): 3407-3419. |
| 22 | Gao K, Guo Z H, Xue C, et al. Effects of biochar and biochar compound fertilizer on the communities of nitrifier and denitrifier in a reclaimed soil from coal-mining area. Chinese Journal of Applied Ecology, 2021, 32(8): 2949-2957. |
| 高科, 郭宗昊, 薛晨, 等. 生物炭与炭基肥对采煤塌陷复垦区土壤硝化和反硝化微生物群落的影响. 应用生态学报, 2021, 32(8): 2949-2957. | |
| 23 | Yang L, Fan M C, Shangguan Z P. An overview of the research in soil nitrogen cycling in rhizosphere. Shaanxi Forest Science and Technology, 2022, 50(5): 116-122. |
| 杨乐, 樊妙春, 上官周平. 根际土壤氮循环过程研究概述. 陕西林业科技, 2022, 50(5): 116-122. | |
| 24 | Yin Y X. Distribution characteristics and impact factors of complete ammonia oxidizing bacteria in bioretentions. Beijing: Peking University, 2021. |
| 银翼翔. 深圳市生物滞留池完全氨氧化微生物的分布及其影响因素研究. 北京: 北京大学, 2021. | |
| 25 | Yu F M, Lin Q J, Wei J Y, et al. Community characteristics of denitrifiers from rhizosphere and bulk soil of plants in the Siding mine area. Journal of Agro-Environment Science. (2023-04-11) [2023-11-16]. http://kns.cnki.net/kcms/detail/12.1347.S.20230410.1856.002.html. |
| 于方明, 林秋娟, 韦嘉裕, 等. 泗顶矿区植物根际和非根际土壤反硝化细菌群落特征. 农业环境科学学报. (2023-04-11) [2023-11-16]. http://kns.cnki.net/kcms/detail/12.1347.S.20230410.1856.002.html. | |
| 26 | Lu Y X, Tao Y, Yin B F, et al. Nitrogen deposition stimulated winter nitrous oxide emissions from bare sand more than biological soil crusts in cold desert ecosystem. Science of the Total Environment, 2022, 841: 156779. |
| 27 | Han S, Luo X S, Liao H, et al. Nitrospira are more sensitive than Nitrobacter to land management in acid, fertilized soils of a rapeseed-rice rotation field trial. Science of the Total Environment, 2017, 599/600: 135-144. |
| 28 | Simek M, Cooper J E, Picek T, et al. Denitrification in arable soils in relation to their physico-chemical properties and fertilization practice. Soil Biology and Biochemistry, 2000, 32(1): 101-110. |
| 29 | Liu X Z. Moso bamboo forest properties and bacterial community diversity of effects of highly intensive management on soil physicochemical. Hangzhou: Zhejiang A&F University, 2021. |
| 刘芯竹. 覆盖经营对毛竹林土壤理化性质和细菌群落多样性影响. 杭州: 浙江农林大学, 2021. | |
| 30 | Lauber C L, Hamady M, Knight R, et al. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Applied and Environmental Microbiology, 2009, 75(15): 5111-5120. |
| 31 | Jiang J S, Yu D, Wang Y, et al. Use of additives in composting informed by experience from agriculture: Effects of nitrogen fertilizer synergists on gaseous nitrogen emissions and corresponding genes (amoA and nirS). Bioresource Technology, 2021, 319: 124127. |
| 32 | Martina K, Henry M, Ramadan E M, et al. Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health. PLoS One, 2011, 6(9): e24452. |
| 33 | Zeng Y, Feng F, Medova H, et al. Functional type 2 photosynthetic reaction centers found in the rare bacterial phylum Gemmatimonadetes. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(21): 7795-7800. |
| 34 | Zhou T T, Hu W G, Zhong Z T, et al. Community composition of nirS-type and nirK-type denitrifying bacteria in rhizosphere of Salicornia europaea in the Ebinur Lake Wetland during different seasons. Acta Ecologica Sinica, 2022, 42(13): 5314-5327. |
| 周婷婷, 胡文革, 钟镇涛, 等. 不同季节艾比湖湿地盐角草根际nirS-型与nirK-型反硝化细菌群落组成分析. 生态学报, 2022, 42(13): 5314-5327. | |
| 35 | Cui H, Sun W, Delgado-Baquerizo M, et al. Phosphorus addition regulates the responses of soil multifunctionality to nitrogen over-fertilization in a temperate grassland. Plant and Soil, 2022, 473(1): 73-87. |
| 36 | Shi Y, Li Y T, Xiang X J, et al. Spatial scale affects the relative role of stochasticity versus determinism in soil bacterial communities in wheat fields across the North China Plain. Microbiome, 2018, 6(1): 27. |
| 37 | Zhang Q, Li Y, He Y, et al. Elevated temperature increased nitrification activity by stimulating AOB growth and activity in an acidic paddy soil. Plant and Soil, 2019, 445: 71-83. |
| [1] | 刘倩, 丁彦芬, 宋杉杉, 许文婕, 杨威. 南京明城墙绿带草本层自生植物群落数量分类与排序分析[J]. 草业学报, 2024, 33(5): 1-15. |
| [2] | 索晓晶, 项磊, 高贺, 运向军, 哈斯巴根, 吴金蕊, 董文成, 滑博伟, 牟金燚, 王琪. 不同利用方式对大针茅草原植被群落特征的影响[J]. 草业学报, 2024, 33(4): 12-21. |
| [3] | 苏尧, 叶苏梅, 鲁梦醒, 马跃, 王玉宝, 王珊珊, 柴如山, 叶新新, 张震, 马超. 整合分析秸秆还田对农田杂草多度和多样性的影响[J]. 草业学报, 2024, 33(3): 150-160. |
| [4] | 雷颖, 罗杰, 郭旭曼, 秘二停, 刘锦春. 小生境尺度下喀斯特弃耕地植物多样性、生物量及其影响因素[J]. 草业学报, 2024, 33(2): 28-38. |
| [5] | 凤紫棋, 孙文义, 穆兴民, 高鹏, 赵广举, 陈帅. 南方山区杉木人工林林下草本植物多样性的影响因素[J]. 草业学报, 2023, 32(9): 17-26. |
| [6] | 吉轶楠, 任雪锋, 苟甜甜, 臧国长, 郑轶琦. 基于SSR标记的河南省假俭草群体遗传多样性研究[J]. 草业学报, 2023, 32(9): 198-212. |
| [7] | 者玉琦, 武志娟, 王吉坤, 钟金城, 柴志欣, 信金伟. 基于mtDNA COX3基因对西藏特色牦牛群体遗传结构的分析[J]. 草业学报, 2023, 32(9): 231-240. |
| [8] | 刘增辉, 卢素锦, 王雨欣, 张春辉, 尹鑫. 三江源地区人工克隆植物群落生物多样性对初级生产力的影响及机制[J]. 草业学报, 2023, 32(9): 27-38. |
| [9] | 赵敏, 赵坤, 王赟博, 殷国梅, 刘思博, 闫宝龙, 孟卫军, 吕世杰, 韩国栋. 长期放牧干扰降低了短花针茅荒漠草原植物多样性[J]. 草业学报, 2023, 32(9): 39-49. |
| [10] | 刘继亮, 赵文智, 王永珍, 冯怡琳, 祁进贤, 李永元. 禁牧和放牧对祁连山高寒草原秋季大型和中型土壤节肢动物多样性的影响[J]. 草业学报, 2023, 32(8): 214-221. |
| [11] | 吕自立, 刘彬, 常凤, 马紫荆, 曹秋梅. 巴音布鲁克高寒草甸物种多样性与系统发育多样性沿海拔梯度分布格局及驱动因子[J]. 草业学报, 2023, 32(7): 12-22. |
| [12] | 刘彩凤, 段媛媛, 王玲玲, 王乙茉, 郭正刚. 高原鼠兔干扰对高寒草甸植物物种多样性与土壤生态化学计量比间关系的影响[J]. 草业学报, 2023, 32(6): 157-166. |
| [13] | 陈彦硕, 马彦平, 王红梅, 赵亚楠, 李志丽, 张振杰. 荒漠草原不同年限灌丛引入过程土壤细菌碳源利用特征[J]. 草业学报, 2023, 32(6): 30-44. |
| [14] | 马婧, 郭方君, 邹枝慧, 孙琳, 陈芳. 腾格里沙漠南缘不同恢复阶段沙质草地植被的季节变化特征[J]. 草业学报, 2023, 32(5): 203-210. |
| [15] | 李美慧, 李玉华, 晏昕辉, 拓行行, 杨梦茹, 王子临, 李伟. 半灌木扩张驱动的草地植物多样性与地上生产力特征及其关系研究[J]. 草业学报, 2023, 32(5): 27-39. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||