

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2021, Vol. 30 ›› Issue (5): 75-83.DOI: 10.11686/cyxb2020505
罗巧玉1,2( ), 王彦龙1, 陈志2, 马永贵2, 任启梅2, 马玉寿1(
), 王彦龙1, 陈志2, 马永贵2, 任启梅2, 马玉寿1( )
)
收稿日期:2020-11-16
修回日期:2021-01-07
出版日期:2021-05-20
发布日期:2021-04-16
通讯作者:
马玉寿
作者简介:Corresponding author. E-mail: mayushou@sina.com基金资助:
Qiao-yu LUO1,2( ), Yan-long WANG1, Zhi CHEN2, Yong-gui MA2, Qi-mei REN2, Yu-shou MA1(
), Yan-long WANG1, Zhi CHEN2, Yong-gui MA2, Qi-mei REN2, Yu-shou MA1( )
)
Received:2020-11-16
Revised:2021-01-07
Online:2021-05-20
Published:2021-04-16
Contact:
Yu-shou MA
摘要:
本研究以发草为研究对象,通过盆栽模拟水分胁迫,研究干旱、水涝胁迫下发草地上部分及根系中脯氨酸(Pro)积累状况及其代谢途径中底物、中间产物和关键酶的变化。结果显示:1)干旱胁迫和水涝胁迫均使发草Pro含量显著升高(P<0.05),相同的水分处理下发草地上部分及根系中Pro含量相差不大。2)干旱胁迫和水涝胁迫下,发草地上部分和根系中谷氨酸(Glu)含量均显著下降(P<0.05),相同的水分处理下根系中Glu含量大于地上部分。水分胁迫使发草地上部分的鸟氨酸(Orn)含量显著下降(P<0.05),而根系中Orn含量没有显著变化。同时,干旱胁迫和水涝胁迫下,发草地上部分和根系中Δ1-吡咯琳-5-羧酸合成酶、鸟氨酸转氨酶(δ-OAT)、Δ1-吡咯琳-5-羧酸还原酶的活性均显著增强(P<0.05),且地上部分δ-OAT活性强于根系。另外,Δ1-吡咯琳-5-羧酸脱氢酶和脯氨酸脱氢酶活性显著降低(P<0.05)。研究结果表明发草通过积累Pro缓解干旱和水涝胁迫,地上部分Pro的积累是Glu途径和Orn途径协同作用的结果,但根系中Pro的积累以Glu途径为主。
罗巧玉, 王彦龙, 陈志, 马永贵, 任启梅, 马玉寿. 水分逆境对发草脯氨酸及其代谢途径的影响[J]. 草业学报, 2021, 30(5): 75-83.
Qiao-yu LUO, Yan-long WANG, Zhi CHEN, Yong-gui MA, Qi-mei REN, Yu-shou MA. Effect of water stress on proline accumulation and metabolic pathways in Deschampsia caespitosa[J]. Acta Prataculturae Sinica, 2021, 30(5): 75-83.
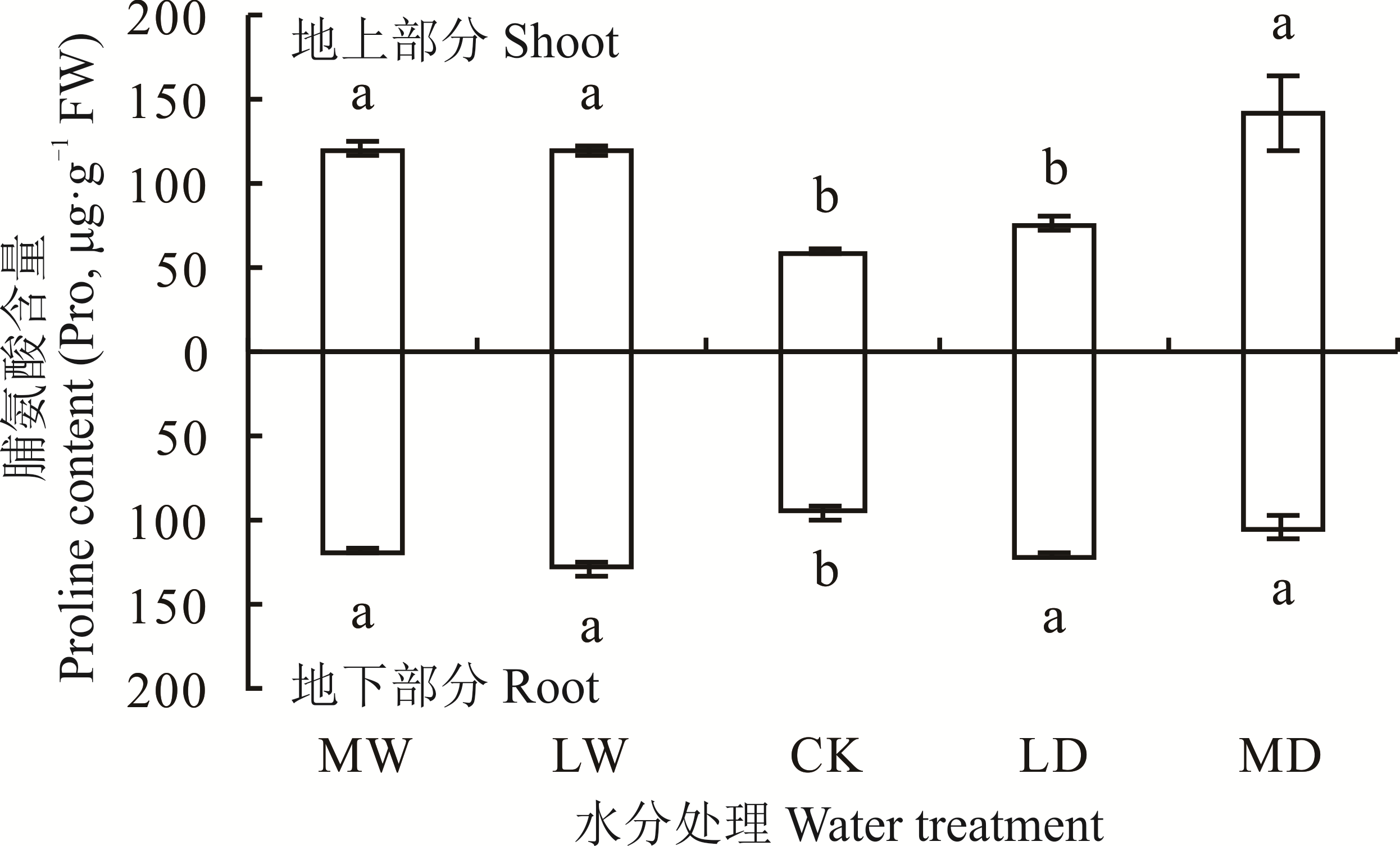
图1 不同水分处理下发草地上/地下部分的Pro含量MW:中度水涝胁迫Medium waterlogging stress; LW:轻度水涝胁迫Light waterlogging stress; CK:对照Control check; LD:轻度干旱胁迫Light dry stress; MD:中度干旱胁迫Medium dry stress。不同小写字母表示不同水分处理下发草地上/地下部分的Pro含量间差异性显著(P<0.05)。Different lowercase letters indicate that there are significant differences among Pro content in the shoot/root of D. caespitosa under different water treatments (P<0.05). 下同The same below.
Fig.1 Proline content in the shoot/root of D. caespitosa under different water treatments
水分处理Water treatment | P5CS | P5CDH | δ-OAT | P5CR | ProDH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | |
| MW | 10.74±0.53a | 7.72±0.34bc | 6.37±0.42ab | 7.26±0.17b | 22.22±1.82ab | 23.76±0.94a | 15.09±1.06a | 5.36±0.18ab | 1.41±0.06a | 2.06±0.07a |
| LW | 11.37±0.61a | 10.39±0.29a | 7.44±0.28ab | 6.57±0.51b | 28.91±3.04a | 20.87±0.44b | 13.89±0.55ab | 6.00±0.20a | 1.33±0.04a | 1.84±0.06a |
| CK | 8.77±0.17b | 6.84±0.09c | 9.83±2.24a | 8.96±0.45a | 20.85±0.56b | 13.19±0.81d | 11.35±0.49c | 4.59±0.23c | 1.43±0.01a | 2.00±0.15a |
| LD | 7.98±0.38b | 7.46±0.53bc | 5.61±0.25b | 7.03±0.28b | 27.93±2.34a | 18.27±0.87c | 11.96±0.23bc | 5.56±0.18a | 1.11±0.04b | 1.92±0.11a |
| MD | 12.32±0.93a | 7.98±0.25b | 8.14±1.44ab | 7.24±0.29b | 24.58±1.36ab | 17.81±0.56c | 15.07±0.92a | 4.90±0.21bc | 1.07±0.08b | 2.10±0.13a |
表1 不同水分处理下发草地上/地下部分Pro代谢关键酶的活性
Table 1 Activity of key enzymes in Pro metabolism in the shoot/root of D. caespitosa under different water treatments (U·g-1 FW)
水分处理Water treatment | P5CS | P5CDH | δ-OAT | P5CR | ProDH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | 地上部分 Shoot | 地下部分 Root | |
| MW | 10.74±0.53a | 7.72±0.34bc | 6.37±0.42ab | 7.26±0.17b | 22.22±1.82ab | 23.76±0.94a | 15.09±1.06a | 5.36±0.18ab | 1.41±0.06a | 2.06±0.07a |
| LW | 11.37±0.61a | 10.39±0.29a | 7.44±0.28ab | 6.57±0.51b | 28.91±3.04a | 20.87±0.44b | 13.89±0.55ab | 6.00±0.20a | 1.33±0.04a | 1.84±0.06a |
| CK | 8.77±0.17b | 6.84±0.09c | 9.83±2.24a | 8.96±0.45a | 20.85±0.56b | 13.19±0.81d | 11.35±0.49c | 4.59±0.23c | 1.43±0.01a | 2.00±0.15a |
| LD | 7.98±0.38b | 7.46±0.53bc | 5.61±0.25b | 7.03±0.28b | 27.93±2.34a | 18.27±0.87c | 11.96±0.23bc | 5.56±0.18a | 1.11±0.04b | 1.92±0.11a |
| MD | 12.32±0.93a | 7.98±0.25b | 8.14±1.44ab | 7.24±0.29b | 24.58±1.36ab | 17.81±0.56c | 15.07±0.92a | 4.90±0.21bc | 1.07±0.08b | 2.10±0.13a |
| 指标Indicator | Pro | Glu | Orn | GSA | P5C | P5CS | P5CDH | P5CR | ProDH |
|---|---|---|---|---|---|---|---|---|---|
| Glu | 0.363 | ||||||||
| Orn | -0.024 | -0.088 | |||||||
| GSA | 0.076 | -0.008 | 0.904** | ||||||
| P5C | -0.352 | -0.518* | 0.641* | 0.491 | |||||
| P5CS | 0.686** | 0.599* | -0.102 | 0.035 | -0.453 | ||||
| P5CDH | 0.517* | 0.566* | 0.154 | 0.215 | -0.141 | 0.502 | |||
| P5CR | 0.808** | 0.404 | -0.243 | -0.103 | -0.353 | 0.654** | 0.521* | ||
| ProDH | -0.235 | 0.593* | -0.305 | -0.289 | -0.512 | 0.010 | -0.049 | -0.200 | |
| δ-OAT | 0.194 | -0.011 | 0.615* | 0.550* | 0.606* | 0.061 | 0.497 | 0.094 | -0.452 |
表2 发草地上部分Pro代谢中各代谢物、关键酶之间的相关性
Table 2 Correlation analysis of metabolites and key enzymes in Pro metabolism in the shoot of D. caespitosa
| 指标Indicator | Pro | Glu | Orn | GSA | P5C | P5CS | P5CDH | P5CR | ProDH |
|---|---|---|---|---|---|---|---|---|---|
| Glu | 0.363 | ||||||||
| Orn | -0.024 | -0.088 | |||||||
| GSA | 0.076 | -0.008 | 0.904** | ||||||
| P5C | -0.352 | -0.518* | 0.641* | 0.491 | |||||
| P5CS | 0.686** | 0.599* | -0.102 | 0.035 | -0.453 | ||||
| P5CDH | 0.517* | 0.566* | 0.154 | 0.215 | -0.141 | 0.502 | |||
| P5CR | 0.808** | 0.404 | -0.243 | -0.103 | -0.353 | 0.654** | 0.521* | ||
| ProDH | -0.235 | 0.593* | -0.305 | -0.289 | -0.512 | 0.010 | -0.049 | -0.200 | |
| δ-OAT | 0.194 | -0.011 | 0.615* | 0.550* | 0.606* | 0.061 | 0.497 | 0.094 | -0.452 |
| 指标Indicator | Pro | Glu | Orn | GSA | P5C | P5CS | P5CDH | P5CR | ProDH |
|---|---|---|---|---|---|---|---|---|---|
| Glu | -0.144 | ||||||||
| Orn | 0.159 | -0.656** | |||||||
| GSA | 0.484 | -0.389 | 0.520* | ||||||
| P5C | 0.370 | -0.773** | 0.806** | 0.626* | |||||
| P5CS | 0.512 | -0.514 | 0.193 | 0.650** | 0.433 | ||||
| P5CDH | -0.672** | -0.016 | -0.214 | -0.777** | -0.599* | -0.114 | |||
| P5CR | 0.507 | -0.537* | 0.699** | 0.815** | 0.645** | 0.508 | -0.468 | ||
| ProDH | -0.238 | 0.494 | -0.331 | -0.350 | -0.296 | -0.368 | -0.045 | -0.367 | |
| δ-OAT | -0.146 | -0.606* | 0.546* | 0.432 | 0.488 | 0.493 | 0.443 | 0.357 | -0.337 |
表3 发草地下部分Pro代谢中各代谢物、关键酶之间的相关性
Table 3 Correlation analysis of metabolites and key enzymes in Pro metabolism in the root of D. caespitosa
| 指标Indicator | Pro | Glu | Orn | GSA | P5C | P5CS | P5CDH | P5CR | ProDH |
|---|---|---|---|---|---|---|---|---|---|
| Glu | -0.144 | ||||||||
| Orn | 0.159 | -0.656** | |||||||
| GSA | 0.484 | -0.389 | 0.520* | ||||||
| P5C | 0.370 | -0.773** | 0.806** | 0.626* | |||||
| P5CS | 0.512 | -0.514 | 0.193 | 0.650** | 0.433 | ||||
| P5CDH | -0.672** | -0.016 | -0.214 | -0.777** | -0.599* | -0.114 | |||
| P5CR | 0.507 | -0.537* | 0.699** | 0.815** | 0.645** | 0.508 | -0.468 | ||
| ProDH | -0.238 | 0.494 | -0.331 | -0.350 | -0.296 | -0.368 | -0.045 | -0.367 | |
| δ-OAT | -0.146 | -0.606* | 0.546* | 0.432 | 0.488 | 0.493 | 0.443 | 0.357 | -0.337 |
| 1 | Hou M J, Gao J L, Ge J, et al. An analysis of dynamic changes and their driving factors in marsh wetlands in the Eastern Qinghai-Tibet Plateau. Acta Prataculturae Sinica, 2020, 29(1): 13-27. |
| 侯蒙京, 高金龙, 葛静, 等. 青藏高原东部高寒沼泽湿地动态变化及其驱动因素研究. 草业学报, 2020, 29(1): 13-27. | |
| 2 | Brierley G J, Li X L, Cullum C, et al. Wetland and its degradation in the Yellow River Source Zone. Springer Geography, 2016(10): 209-232. |
| 3 | Duggan E M F, Pagès J F, Jenkins S R, et al. External conditions drive optimal planting configurations for salt marsh restoration. Journal of Applied Ecology, 2020, 57(3): 619-629. |
| 4 | Brisson J, Rodriguez M, Martin C A, et al. Plant diversity effect on water quality in wetlands: A meta-analysis based on experimental systems. Ecological Applications, 2020, 30(4): e02074. |
| 5 | Zhu Y J, Ma M Y, Zhao N N. Progress and prospect of restoration technology of degraded alpine peatlands in Zoige Plateau. Chinese Journal of Ecology, 2020, 39(12): 4185-4192. |
| 朱耀军, 马牧源, 赵娜娜. 若尔盖高寒泥炭地修复技术进展与展望. 生态学杂志, 2020, 39(12): 4185-4192. | |
| 6 | Wang Y L, Ma Y S, Shi J J, et al. Study on cultivation and domestication of Deschampsia caespitosa. Chinese Qinghai Journal of Animal and Veterinary Sciences, 2019, 49(2): 21-24. |
| 王彦龙, 马玉寿, 施建军, 等. 发草栽培驯化研究初报. 青海畜牧兽医杂志, 2019, 49(2): 21-24. | |
| 7 | Luo Q Y, Wang Y L, Du L, et al. Plant community diversity and soil factor interpretation of adaptive region of Deschampsia caespitosa in source region of Yellow River. Acta Prataculturae Sinica, 2021, 30(4): 80-89. |
| 罗巧玉, 王彦龙, 杜雷, 等. 黄河源区发草适生地植物群落特征及其土壤因子解释. 草业学报, 2021, 30(4):80-89. | |
| 8 | Li H L, Li X L, Zhou X L. Trait means predict performance under water limitation better than plasticity for seedlings of Poaceae species on the Eastern Tibetan Plateau. Ecology and Evolution, 2020, 10: 2944-2955. |
| 9 | Wang H X. The study of vegetation community characteristics and evaluation of LUCC in the semi-arid wetland. Beijing: Beijing Forestry University, 2012. |
| 王海星. 西北半干旱区湿地植被群落特征研究及其LUCC评价体系构建. 北京: 北京林业大学, 2012. | |
| 10 | Das S K, Patra J K, Thatoi H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. International Review of Hydrobiology, 2016, 101(1/2): 3-19. |
| 11 | Li B Z, Zhou G S. Advance in the study on drought index. Acta Ecologica Sinica, 2014, 34(5): 1043-1052. |
| 李柏贞, 周广胜. 干旱指标研究进展. 生态学报, 2014, 34(5): 1043-1052. | |
| 12 | Nadeem M, Li J J, Yahya M, et al. Research progress and perspective on drought stress in legumes: A review. International Journal of Molecular Sciences, 2019, 20(10): 2541. |
| 13 | Khan M S, Ahmad D, Khan M A. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electronic Journal of Biotechnology, 2015, 18(4): 257-266. |
| 14 | Bian W J, Bao G Z, Qian H M, et al. Physiological response characteristics in Medicago sativa under freeze-thaw and deicing salt stress.Water, Air & Soil Pollution, 2018, 229(6): 196. |
| 15 | Wang K Y, Chen F Q, Huang W X. Research advance on drought stress response mechanism in plants. Journal of Agricultural Science and Technology, 2019, 21(2): 19-25. |
| 王凯悦, 陈芳泉, 黄五星. 植物干旱胁迫响应机制研究进展. 中国农业科技导报, 2019, 21(2): 19-25. | |
| 16 | Li K, Zhou Z Y, Li S J,et al. Growth, osmotic adjustment and antioxidant capacity responses of Schizomepeta tenuifolia to drought stress. Acta Prataculturae Sinica, 2020, 29(5): 150-158. |
| 李柯, 周庄煜, 李四菊, 等. 荆芥的生长、渗透调节和抗氧化能力对干旱胁迫的响应. 草业学报, 2020, 29(5): 150-158. | |
| 17 | Boyce R L, Durtsche R D. Plant colonization of a restored wetland in Northern Kentucky: Contribution of seeding vs. natural sources. The Journal of the Torrey Botanical Society, 2020, 147(1): 9-21. |
| 18 | Zeng L S, Li P Y, Sun X F, et al. A multi-trait evaluation of drought resistance of bermudagrass (Cynodon dactylon) germplasm from different habitats in Xinjiang Province. Acta Prataculturae Sinica, 2020, 29(8): 155-169. |
| 曾令霜, 李培英, 孙晓梵, 等. 新疆不同生境狗牙根种质抗旱性综合评价. 草业学报, 2020, 29(8): 155-169. | |
| 19 | Tuo X Q, Li S, Wu Q S, et al. Alleviation of waterlogged stress in peach seedlings inoculated with Funneliformis mosseae: Changes in chlorophyll and proline metabolism. Scientia Horticulturae, 2015, 197: 130-134. |
| 20 | Das B, Padhiary A K, Behera S, et al. Biochemical changes in some rice varieties in response to waterlogged and submerged Conditions. International Journal of Pure & Applied Bioscience, 2017, 5(5): 972-978. |
| 21 | Jia Y, Xiang Y F, Wang L L, et al. Effects of salt stress on the growth and physiological characteristics of Primula forbesii. Acta Prataculturae Sinica, 2020, 29(10): 119-128. |
| 贾茵, 向元芬, 王琳璐, 等. 盐胁迫对小报春生长及生理特性的影响. 草业学报, 2020, 29(10): 119-128. | |
| 22 | Zhong H, Dong J, Dong K H. Effect of salt stress on proline accumulation and the activities of the key enzymes involved in proline metabolism in Medicago ruthenica seedlings. Acta Prataculturae Sinica, 2018, 27(4): 189-194. |
| 钟华, 董洁, 董宽虎. 盐胁迫对扁蓿豆幼苗脯氨酸积累及其代谢关键酶活性的影响. 草业学报, 2018, 27(4): 189-194. | |
| 23 | Moukhtari A E, Cabassa-Hourton C, Farissi M, et al. How does proline treatment promote salt stress tolerance during crop plant development? Frontiers in Plant Science, 2020, 11: 1127. |
| 24 | Bao G Z, Ao Q, Li Q Q, et al. Physiological characteristics of Medicago sativa L. in response to acid deposition and freeze-thaw stress. Water, Air, & Soil Pollution, 2017, 228(9): 376. |
| 25 | Chang Y X, Bao G Z, Zhang M Y. Effects of drought and freeze thaw stress on antioxidant enzymes activities and mass ratio of proline of Secale cereal L. Journal of Jilin University (Science Edition), 2020, 58(1): 184-188. |
| 常艺馨, 包国章, 张梦瑜. 干旱及冻融胁迫对黑麦草抗氧化酶活性和脯氨酸质量比的影响. 吉林大学学报(理学版), 2020, 58(1): 184-188. | |
| 26 | Shi Z Z, Li S, Ma S Y, et al. Response of the antioxidant system to water stress in different wheat varieties. Acta Prataculturae Sinica, 2015, 24(7): 68-78. |
| 时振振, 李胜, 马绍英, 等. 不同品种小麦抗氧化系统对水分胁迫的响应. 草业学报, 2015, 24(7): 68-78. | |
| 27 | Zhu G C. Physiological ecological studies on adaptation to water stress in Leersia hexandra as a drought-flood co-tolerant plant. Guangzhou: Sun Yat-Sen University, 2011. |
| 朱桂才. 共耐性植物李氏禾(Leersia hexandra)的水分逆境生理生态适应机制研究. 广州: 中山大学, 2011. | |
| 28 | Ogbaga C C, Stepien P, Johnson G N. Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Physiologia Plantarum, 2014, 152(2): 389-401. |
| 29 | Gao J F. Plant physiology experiment guidance. Beijing: Higher Education Press, 2006: 15-16, 228-231. |
| 高俊凤. 植物生理学实验指导. 北京: 高等教育出版社, 2006: 15-16, 228-231. | |
| 30 | Lutts S, Majerus V, Kinet J M. NaCl effects on proline metabolism in rice (Orya satica) seedlings. Physiologia Plantarum, 1999, 105(3): 450-458. |
| 31 | Garcia-Rios M, Fujita T, Larosa P C, et al. Cloning of a polycistronic cDNA from tomato encoding γ-glutamyl kinase and γ-glutamyl phosphate reductase. Proceedings of the National Academy of Sciences, 1997, 94(15): 8249-8254. |
| 32 | Charest C, Phan C T. Cold acclimation of wheat (Triticum aestioum): Properties of enzymes involved in proline metabolism. Physiologia Plantarum, 1990, 80(2): 159-168. |
| 33 | Ren Y, Miao M, Meng Y, et al. DFR1-mediated inhibition of proline degradation pathway regulates drought and freezing tolerance in Arabidopsis. Cell Reports, 2018, 23(13): 3960-3974. |
| 34 | Su J C, Zhang Y H, Nie Y, et al. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environmental and Experimental Botany, 2018, 147: 249-260. |
| 35 | Wani A S, Ahmad A, Hayat S, et al. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiology and Biochemistry, 2019, 135: 385-394. |
| 36 | Zouari M, Ahmed C B, Zorrig W, et al. Exogenous proline mediates alleviation of cadmium stress by promoting photosynthetic activity, water status and antioxidative enzymes activities of young date palm (Phoenix dactylifera L.). Ecotoxicology & Environmental Safety, 2016, 128: 100-108. |
| 37 | Teh C Y, Ho C L, Shaharuddin N A, et al. Proteome of rice roots treated with exogenous proline. Biotech, 2019, 9(3): 110. |
| 38 | Li D Y, Yan Y Q, Yin Y, et al. Effects of Spd and NO on proline metabolic pathways of Polygonatum odoratum (Mill.) druce under salt stress. Journal of Henan Agricultural Sciences, 2018, 47(6): 111-116. |
| 李丹阳, 闫永庆, 殷媛, 等. 外源Spd和NO对盐胁迫下玉竹脯氨酸代谢途径的影响. 河南农业科学, 2018, 47(6): 111-116. | |
| 39 | Sikder R K, Wang X R, Zhang H H, et al. Nitrogen enhances salt tolerance by modulating the antioxidant defense system and osmoregulation substance content in Gossypium hirsutum. Plant, 2020, 9(4): 450. |
| 40 | Zhao Y, Wei X H, Li T T. Effects of exogenous nitric oxide on seed germination and seeding growth of Chenopodium quinoa under complex saline-alkali stress. Acta Prataculturae Sinica, 2020, 29(4): 92-101. |
| 赵颖, 魏小红, 李桃桃. 外源NO对混合盐碱胁迫下藜麦种子萌发和幼苗生长的影响. 草业学报, 2020, 29(4): 92-101. |
| [1] | 臧真凤, 白婕, 刘丛, 昝看卓, 龙明秀, 何树斌. 紫花苜蓿形态和生理指标响应干旱胁迫的品种特异性[J]. 草业学报, 2021, 30(6): 73-81. |
| [2] | 候怡谣, 李霄, 龙瑞才, 杨青川, 康俊梅, 郭长虹. 过量表达紫花苜蓿MsHB7基因对拟南芥耐旱性的影响[J]. 草业学报, 2021, 30(4): 170-179. |
| [3] | 罗巧玉, 王彦龙, 杜雷, 刘念, 李丽, 马玉寿. 黄河源区发草适生地植物群落特征及其土壤因子解释[J]. 草业学报, 2021, 30(4): 80-89. |
| [4] | 刘凯强, 刘文辉, 贾志锋, 梁国玲, 马祥. 干旱胁迫对‘青燕1号’燕麦产量及干物质积累与分配的影响[J]. 草业学报, 2021, 30(3): 177-188. |
| [5] | 李冬, 申洪涛, 王艳芳, 王悦华, 王丽君, 赵世民, 刘领. 外源褪黑素对干旱胁迫下烟草幼苗光合碳同化和内源激素的影响[J]. 草业学报, 2021, 30(1): 130-139. |
| [6] | 曾令霜, 李培英, 孙晓梵, 孙宗玖. 新疆不同生境狗牙根种质抗旱性综合评价[J]. 草业学报, 2020, 29(8): 155-169. |
| [7] | 张宇君, 尚以顺, 王普昶, 丁磊磊, 张文, 邹超. 干旱胁迫下保水剂对盘江白刺花幼苗生长和生理特性的影响[J]. 草业学报, 2020, 29(7): 90-98. |
| [8] | 王泳超, 张颖蕾, 闫东良, 何灵芝, 李卓, 燕博文, 邵瑞鑫, 郭家萌, 杨青华. 干旱胁迫下γ-氨基丁酸保护玉米幼苗光合系统的生理响应[J]. 草业学报, 2020, 29(6): 191-203. |
| [9] | 李柯, 周庄煜, 李四菊, 姚浩铮, 周莹, 缪雨静, 唐晓清, 王康才. 荆芥的生长、渗透调节和抗氧化能力对干旱胁迫的响应[J]. 草业学报, 2020, 29(5): 150-158. |
| [10] | 高子奇, 王佳, 汤宇晨, 王迎春. 唐古特白刺类黄酮-3-O-葡萄糖基转移酶基因(NtUFGT)的克隆与功能分析[J]. 草业学报, 2020, 29(5): 159-170. |
| [11] | 赵小强, 陆晏天, 白明兴, 徐明霞, 彭云玲, 丁永福, 庄泽龙, 陈奋奇, 张大志. 不同株型玉米基因型对干旱胁迫的响应分析[J]. 草业学报, 2020, 29(2): 149-162. |
| [12] | 何建军, 姚立蓉, 汪军成, 边秀秀, 司二静, 杨轲, 王化俊, 马小乐, 李葆春, 尚勋武, 孟亚雄. 干旱和盐胁迫对盐生植物盐生草种子萌发特性的影响[J]. 草业学报, 2020, 29(11): 129-140. |
| [13] | 许爱云, 曹兵, 谢云. 干旱风沙区煤炭基地12种草本植物对干旱胁迫的生理生态响应及抗旱性评价[J]. 草业学报, 2020, 29(10): 22-34. |
| [14] | 马碧花, 蔺伟虎, 高敏, 王兴迪, 田沛. 干旱胁迫下水杨酸和内生真菌对多年生黑麦草的影响[J]. 草业学报, 2020, 29(1): 135-144. |
| [15] | 刘领, 李冬, 马宜林, 王丽君, 赵世民, 周俊学, 申洪涛, 王艳芳. 外源褪黑素对干旱胁迫下烤烟幼苗生长的缓解效应与生理机制研究[J]. 草业学报, 2019, 28(8): 95-105. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||