

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2021, Vol. 30 ›› Issue (11): 170-180.DOI: 10.11686/cyxb2020562
• 研究论文 • 上一篇
吴晨( ), 姚志浩, 梅文晴, 冯宇妍, 陈渠, 倪迎冬(
), 姚志浩, 梅文晴, 冯宇妍, 陈渠, 倪迎冬( )
)
收稿日期:2020-12-15
修回日期:2021-03-15
出版日期:2021-10-19
发布日期:2021-10-19
通讯作者:
倪迎冬
作者简介:Corresponding author. E-mail: niyingdong@njau.edu.cn基金资助:
Chen WU( ), Zhi-hao YAO, Wen-qing MEI, Yu-yan FENG, Qu CHEN, Ying-dong NI(
), Zhi-hao YAO, Wen-qing MEI, Yu-yan FENG, Qu CHEN, Ying-dong NI( )
)
Received:2020-12-15
Revised:2021-03-15
Online:2021-10-19
Published:2021-10-19
Contact:
Ying-dong NI
摘要:
本试验旨在研究日粮中添加复合维生素B对生长期山羊消化道微生物组成和肠黏膜结构的影响。试验选取10只4月龄波杂雌山羊(12.60±1.28) kg,随机分为对照组(control,CON)和复合维生素B处理组(vitamin B,VB),CON组饲喂基础日粮(n=5),VB组在基础日粮中添加复合维生素B(n=5),预饲期2周,试验期11周,试验期间自由饮水。试验结束后屠宰采样,16S rRNA测序分析盲肠和结肠内容物微生物组成,检测肠道上皮组织形态结构和相关基因与蛋白的表达。通过菌群分类学分析发现,与CON相比,VB组山羊盲肠和结肠内容物菌群的α多样性指数无显著变化(P>0.05);在门水平上,盲肠和结肠内容物菌群中厚壁菌门(Firmicutes)和拟杆菌门(Bacteroidetes)为优势菌门,VB组盲肠拟杆菌门丰度有升高趋势(0.05<P<0.1),而螺旋菌门(Spirochaetes)丰度有降低趋势(0.05<P<0.1);在目水平上,VB组盲肠Solirubrobacterales和Streptomycetales目菌群丰度显著下降(P<0.05);在科水平上,VB组盲肠Christensenellaceae、Streptomycetaceae、Solirubrobacteraceae及结肠Christensenellaceae科菌群丰度显著下降(P<0.05);在属水平上,VB组盲肠Lachnoclostridium属菌群丰度显著升高(P<0.05),盲肠Bacillus、Nocardioides及结肠unidentified_Christensenellaceae和unidentified_Gammaproteobacteria属菌群丰度显著下降(P<0.05)。与CON相比,VB组山羊空肠绒毛高度(P<0.01)、隐窝深度(P<0.05)和绒毛高度/隐窝深度(V/C,P<0.05)均显著增加;回肠隐窝深度显著降低(P<0.05),绒毛高度和V/C无显著变化(P>0.05)。实时荧光定量PCR结果显示,与CON相比,VB 组山羊盲肠上皮紧密连接和细胞增殖相关基因的表达均无显著变化(P>0.05);而结肠黏膜上皮Occludin(P<0.05)、EGFR(P<0.01)、MIK67(P<0.05)和CCND(P<0.05)基因表达均显著上调;且VB组ZO-1蛋白表达显著上调(P<0.05)。综上表明,日粮中添加复合维生素B对生长期山羊肠道菌群多样性无显著影响,但可提高肠道中有益菌的丰度且降低有害菌的丰度,从而促进肠道绒毛的生长及紧密连接蛋白的表达,对山羊肠道健康和生长具有积极作用。
吴晨, 姚志浩, 梅文晴, 冯宇妍, 陈渠, 倪迎冬. 复合维生素B对生长期山羊后段肠道菌群组成及肠黏膜的影响[J]. 草业学报, 2021, 30(11): 170-180.
Chen WU, Zhi-hao YAO, Wen-qing MEI, Yu-yan FENG, Qu CHEN, Ying-dong NI. Effects of vitamin B complex on intestinal microflora composition and gut epithelial structure in growing goats[J]. Acta Prataculturae Sinica, 2021, 30(11): 170-180.
项目 Item | 原料 Ingredients (%) | 项目 Item | 营养水平 Nutrient levels2 ) |
|---|---|---|---|
| 精粗比The ratio of concentrate to forage | 40∶60 | 消化能Digestible energy (MJ·kg-1) | 8.25 |
| 苜蓿青贮Alfalfa silage | 60 | 粗蛋白Crude protein (%) | 18.05 |
| 玉米 Corn | 22.4 | 粗脂肪Ether extract (%) | 3.00 |
| 麸皮Wheat bran | 4.8 | 中性洗涤纤维Neutral detergent fiber (%) | 35.07 |
| 豆粕 Soybean meal | 10.8 | 酸性洗涤纤维Acid detergent fiber (%) | 24.50 |
| 小苏打Baking soda | 0.4 | 钙Calcium (%) | 0.88 |
| 预混料 Premix1) | 1.6 | 总磷Total phosphorus (%) | 0.35 |
| 总计 Total | 100 |
表1 日粮组成及营养水平
Table 1 Dietary composition and nutrient levels
项目 Item | 原料 Ingredients (%) | 项目 Item | 营养水平 Nutrient levels2 ) |
|---|---|---|---|
| 精粗比The ratio of concentrate to forage | 40∶60 | 消化能Digestible energy (MJ·kg-1) | 8.25 |
| 苜蓿青贮Alfalfa silage | 60 | 粗蛋白Crude protein (%) | 18.05 |
| 玉米 Corn | 22.4 | 粗脂肪Ether extract (%) | 3.00 |
| 麸皮Wheat bran | 4.8 | 中性洗涤纤维Neutral detergent fiber (%) | 35.07 |
| 豆粕 Soybean meal | 10.8 | 酸性洗涤纤维Acid detergent fiber (%) | 24.50 |
| 小苏打Baking soda | 0.4 | 钙Calcium (%) | 0.88 |
| 预混料 Premix1) | 1.6 | 总磷Total phosphorus (%) | 0.35 |
| 总计 Total | 100 |
基因 Gene | 引物序列 Primer sequences (5′—3′) | 基因序列号 GenBank accession No. |
|---|---|---|
| CCND | R:AAAGCCCTCTTCCATACA F:CTCCGCCTTCCCTAAC | XM_005680985.1 |
| MKI67 | R:TCAGTGAGCAGGAGGCAGTA F:GGAAATCCAGGTGACTTGCT | XM_004004769.1 |
| Claudin 1 | R:CACCCTTGGCATGAAGTGTA F:AGCCAATGAAGAGAGCCTGA | HM117762.1 |
| Claudin 4 | R:AAGGTGTACGACTCGCTGCT F:GACGTTGTTAGCCGTCCAG | HM117763.1 |
| Occludin | R:GTTCGACCAATGCTCTCTCAG F:CAGCTCCCATTAAGGTTCCA | BC133617.1 |
| EGFR | R:AACTGTGAGGTGGTCCTTGG F:CACTGTGTTGAGGGCAATGA | XM_005695500.1 |
| GAPDH | R:GGGTCATCATCTCTGCACCT F:GGTCATAAGTCCCTCCACGA | HM043737.1 |
表2 实时荧光定量PCR引物序列
Table 2 Real-time fluorescence quantitative PCR primer sequences
基因 Gene | 引物序列 Primer sequences (5′—3′) | 基因序列号 GenBank accession No. |
|---|---|---|
| CCND | R:AAAGCCCTCTTCCATACA F:CTCCGCCTTCCCTAAC | XM_005680985.1 |
| MKI67 | R:TCAGTGAGCAGGAGGCAGTA F:GGAAATCCAGGTGACTTGCT | XM_004004769.1 |
| Claudin 1 | R:CACCCTTGGCATGAAGTGTA F:AGCCAATGAAGAGAGCCTGA | HM117762.1 |
| Claudin 4 | R:AAGGTGTACGACTCGCTGCT F:GACGTTGTTAGCCGTCCAG | HM117763.1 |
| Occludin | R:GTTCGACCAATGCTCTCTCAG F:CAGCTCCCATTAAGGTTCCA | BC133617.1 |
| EGFR | R:AACTGTGAGGTGGTCCTTGG F:CACTGTGTTGAGGGCAATGA | XM_005695500.1 |
| GAPDH | R:GGGTCATCATCTCTGCACCT F:GGTCATAAGTCCCTCCACGA | HM043737.1 |
| 项目 Items | 指标 Index | 对照组 CON | VB组 VB | P值P-value |
|---|---|---|---|---|
盲肠内容物 Cecal digesta | ACE指数 ACE | 1649.32±108.40 | 1525.63±105.40 | 0.45 |
| Chao 1指数 Chao 1 | 1672.20±130.08 | 1521.72±132.24 | 0.45 | |
| 香农指数 Shannon index | 7.87±0.12 | 7.67±0.04 | 0.15 | |
结肠内容物 Colonic digesta | ACE指数ACE | 1383.60±106.60 | 1383.32±42.36 | 1.00 |
| Chao 1指数Chao 1 | 1343.51±104.90 | 1355.62±33.51 | 0.92 | |
| 香农指数Shannon index | 7.70±0.15 | 7.42±0.18 | 0.27 |
表3 山羊肠道微生物α多样性分析
Table 3 Alpha diversity analysis of intestinal microbes in goats (n=4)
| 项目 Items | 指标 Index | 对照组 CON | VB组 VB | P值P-value |
|---|---|---|---|---|
盲肠内容物 Cecal digesta | ACE指数 ACE | 1649.32±108.40 | 1525.63±105.40 | 0.45 |
| Chao 1指数 Chao 1 | 1672.20±130.08 | 1521.72±132.24 | 0.45 | |
| 香农指数 Shannon index | 7.87±0.12 | 7.67±0.04 | 0.15 | |
结肠内容物 Colonic digesta | ACE指数ACE | 1383.60±106.60 | 1383.32±42.36 | 1.00 |
| Chao 1指数Chao 1 | 1343.51±104.90 | 1355.62±33.51 | 0.92 | |
| 香农指数Shannon index | 7.70±0.15 | 7.42±0.18 | 0.27 |

图2 山羊肠道微生物门水平相对丰度A:山羊盲肠微生物门水平相对丰度;B:山羊结肠微生物门水平相对丰度。A: Relative abundance of microbes in cecum; B: Relative abundance of microbes in colon.
Fig.2 Relative abundance of intestinal microbes in goats at phylum level (n=4)
项目 Items | 对照组 CON | VB组 VB | P值 P-value |
|---|---|---|---|
| 厚壁菌门Firmicutes | 74.20±1.91 | 69.75±2.19 | 0.18 |
| 拟杆菌门Bacteroidetes | 13.01±2.40 | 19.23±1.69 | 0.08# |
| 广古菌门Euryarchaeota | 4.35±1.29 | 3.32±1.14 | 0.57 |
| 变形菌门Proteobacteria | 2.59±0.44 | 3.02±0.17 | 0.40 |
| 放线菌门Actinobacteria | 2.19±0.28 | 1.77±0.16 | 0.23 |
| 螺旋菌门Spirochaetes | 1.04±0.18 | 0.48±0.16 | 0.06# |
| 软壁菌门Tenericutes | 0.91±0.19 | 0.88±0.08 | 0.91 |
| 酸杆菌门Acidobacteria | 0.79±0.12 | 0.79±0.25 | 1.00 |
| 疣微菌门Verrucomicrobia | 0.09±0.03 | 0.13±0.08 | 0.60 |
| 绿弯菌门Chloroflexi | 0.16±0.05 | 0.11±0.03 | 0.43 |
表4 山羊盲肠微生物门水平丰度比较
Table 4 Comparison of abundance of cecal microbes in goats at phylum level (n=4)
项目 Items | 对照组 CON | VB组 VB | P值 P-value |
|---|---|---|---|
| 厚壁菌门Firmicutes | 74.20±1.91 | 69.75±2.19 | 0.18 |
| 拟杆菌门Bacteroidetes | 13.01±2.40 | 19.23±1.69 | 0.08# |
| 广古菌门Euryarchaeota | 4.35±1.29 | 3.32±1.14 | 0.57 |
| 变形菌门Proteobacteria | 2.59±0.44 | 3.02±0.17 | 0.40 |
| 放线菌门Actinobacteria | 2.19±0.28 | 1.77±0.16 | 0.23 |
| 螺旋菌门Spirochaetes | 1.04±0.18 | 0.48±0.16 | 0.06# |
| 软壁菌门Tenericutes | 0.91±0.19 | 0.88±0.08 | 0.91 |
| 酸杆菌门Acidobacteria | 0.79±0.12 | 0.79±0.25 | 1.00 |
| 疣微菌门Verrucomicrobia | 0.09±0.03 | 0.13±0.08 | 0.60 |
| 绿弯菌门Chloroflexi | 0.16±0.05 | 0.11±0.03 | 0.43 |
项目 Items | 对照组 CON | VB组 VB | P值 P-value |
|---|---|---|---|
| 厚壁菌门Firmicutes | 72.69±0.77 | 71.70±2.24 | 0.69 |
| 拟杆菌门Bacteroidetes | 14.75±1.52 | 15.96±1.06 | 0.54 |
| 广古菌门Euryarchaeota | 4.82±1.72 | 1.52±0.61 | 0.12 |
| 变形菌门Proteobacteria | 1.95±0.31 | 2.58±0.20 | 0.13 |
| 放线菌门Actinobacteria | 1.44±0.40 | 1.85±0.26 | 0.42 |
| 螺旋菌门Spirochaetes | 2.00±0.78 | 0.48±0.21 | 0.11 |
| 软壁菌门Tenericutes | 0.81±0.10 | 0.58±0.09 | 0.15 |
| 酸杆菌门Acidobacteria | 0.45±0.15 | 0.92±0.23 | 0.14 |
| 疣微菌门Verrucomicrobia | 0.59±0.50 | 3.90±2.49 | 0.24 |
| 绿弯菌门Chloroflexi | 0.08±0.03 | 0.13±0.03 | 0.27 |
表5 山羊结肠微生物门水平丰度比较
Table 5 Comparison of abundance of colonic microbes in goats at phylum level (n=4)
项目 Items | 对照组 CON | VB组 VB | P值 P-value |
|---|---|---|---|
| 厚壁菌门Firmicutes | 72.69±0.77 | 71.70±2.24 | 0.69 |
| 拟杆菌门Bacteroidetes | 14.75±1.52 | 15.96±1.06 | 0.54 |
| 广古菌门Euryarchaeota | 4.82±1.72 | 1.52±0.61 | 0.12 |
| 变形菌门Proteobacteria | 1.95±0.31 | 2.58±0.20 | 0.13 |
| 放线菌门Actinobacteria | 1.44±0.40 | 1.85±0.26 | 0.42 |
| 螺旋菌门Spirochaetes | 2.00±0.78 | 0.48±0.21 | 0.11 |
| 软壁菌门Tenericutes | 0.81±0.10 | 0.58±0.09 | 0.15 |
| 酸杆菌门Acidobacteria | 0.45±0.15 | 0.92±0.23 | 0.14 |
| 疣微菌门Verrucomicrobia | 0.59±0.50 | 3.90±2.49 | 0.24 |
| 绿弯菌门Chloroflexi | 0.08±0.03 | 0.13±0.03 | 0.27 |
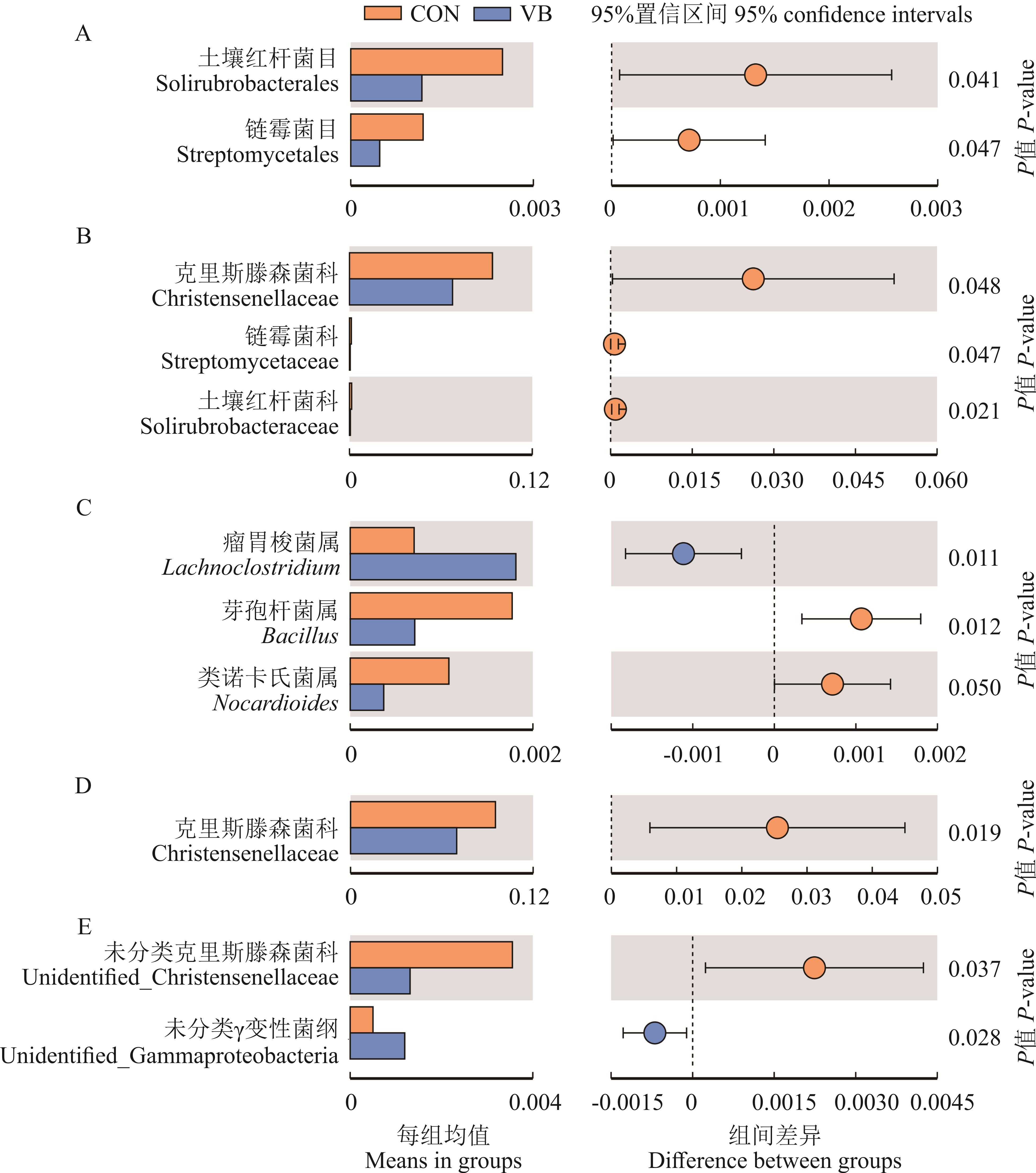
图3 山羊肠道微生物组间差异分析A:盲肠微生物目水平差异分析;B:盲肠微生物科水平差异分析;C:盲肠微生物属水平差异分析;D:结肠微生物科水平差异分析;E:结肠微生物属水平差异分析。A: Analysis of cecum microbes composition at order level; B: Analysis of cecum microbes composition at family level; C: Analysis of cecum microbes composition at genus level; D: Analysis of colon microbes composition at family level; E: Analysis of colon microbes composition at genus level.
Fig.3 Analysis of intestinal microbial differences in goats (n=4)
| 部位 Parts | 项目 Items | 对照组 CON | VB组 VB | P值P-value |
|---|---|---|---|---|
| 空肠 Jejunum | 绒毛高度 Villus height (V, μm) | 493.50±13.42A | 623.74±1.36B | 0.00 |
| 隐窝深度 Crypt depth (C, μm) | 195.24±5.85a | 218.58±6.08b | 0.02 | |
| 绒毛高度/隐窝深度 V/C | 2.53±0.07a | 2.86±0.08b | 0.01 | |
| 回肠 Ileum | 绒毛高度 Villus height (V, μm) | 503.42±36.51 | 505.93±27.21 | 0.96 |
| 隐窝深度 Crypt depth (C, μm) | 197.27±3.00b | 177.38±4.93a | 0.01 | |
| 绒毛高度/隐窝深度 V/C | 2.55±0.18 | 2.86±0.16 | 0.23 |
表6 山羊空肠和回肠组织形态的变化
Table 6 Changes in morphology of jejunum and ileum in goats
| 部位 Parts | 项目 Items | 对照组 CON | VB组 VB | P值P-value |
|---|---|---|---|---|
| 空肠 Jejunum | 绒毛高度 Villus height (V, μm) | 493.50±13.42A | 623.74±1.36B | 0.00 |
| 隐窝深度 Crypt depth (C, μm) | 195.24±5.85a | 218.58±6.08b | 0.02 | |
| 绒毛高度/隐窝深度 V/C | 2.53±0.07a | 2.86±0.08b | 0.01 | |
| 回肠 Ileum | 绒毛高度 Villus height (V, μm) | 503.42±36.51 | 505.93±27.21 | 0.96 |
| 隐窝深度 Crypt depth (C, μm) | 197.27±3.00b | 177.38±4.93a | 0.01 | |
| 绒毛高度/隐窝深度 V/C | 2.55±0.18 | 2.86±0.16 | 0.23 |
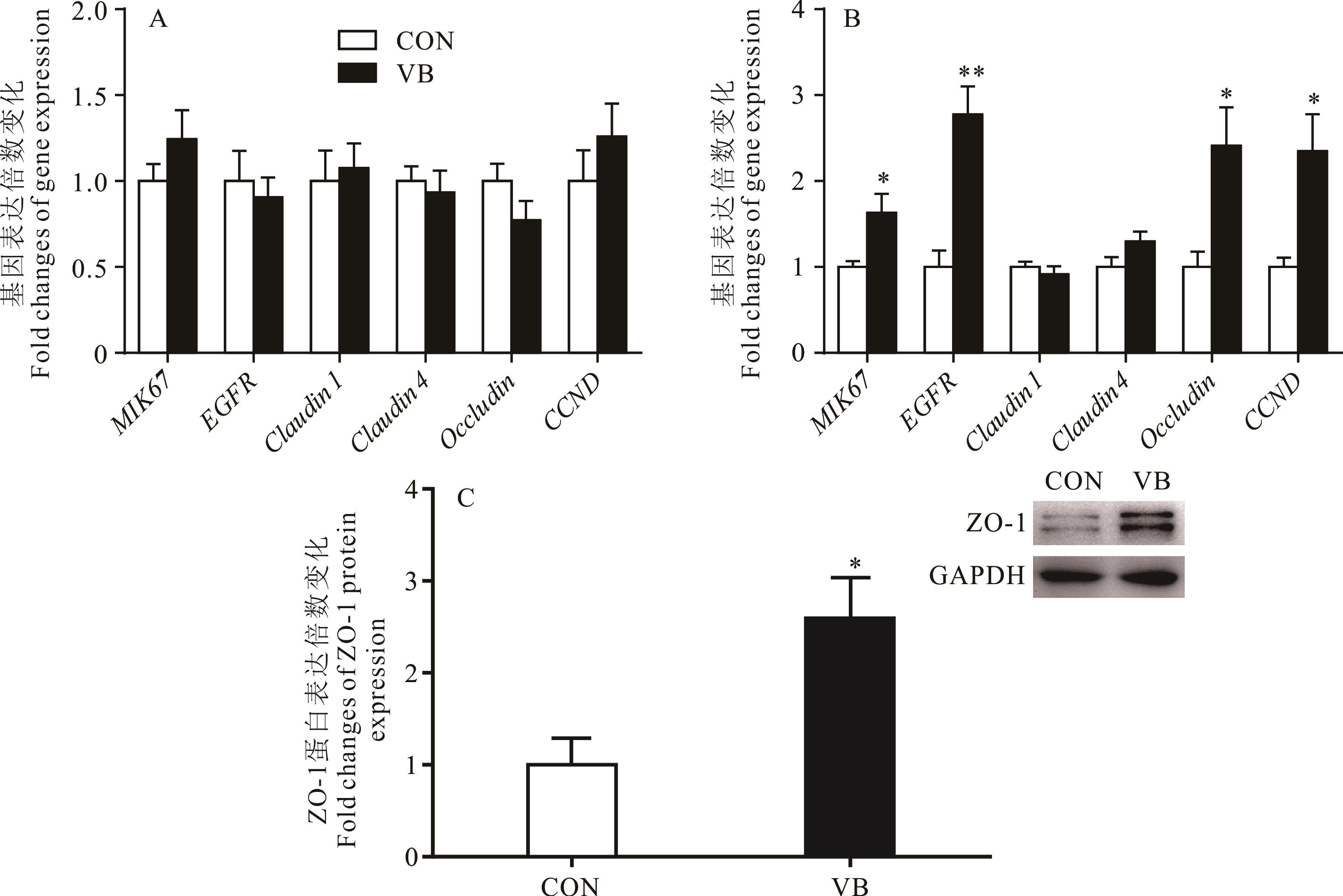
图5 山羊肠道上皮相关基因和蛋白表达变化A:山羊盲肠中相关基因表达;B:山羊结肠中相关基因表达;C:山羊结肠中ZO-1蛋白表达。**表示数据差异极显著(P<0.01);*表示差异显著(P<0.05)。A: Expression of related genes in the cecum of goats; B: Expression of related genes in the colon of goats; C: Expression of ZO-1 protein in the colon of goats. ** indicates that the difference is extremely significant (P<0.01); * indicates that the difference is significant (P<0.05).
Fig.5 Changes in genes and proteins expression in intestinal epithelium of goats (n=5)
| 1 | Beaudet V, Gervais R, Graulet B, et al. Effects of dietary nitrogen levels and carbohydrate sources on apparent ruminal synthesis of some B vitamins in dairy cows. Journal of Dairy Science, 2016, 99(4): 2730-2739. |
| 2 | Hooper L V, Gordon J I. Commensal host-bacterial relationships in the gut. Science, 2001, 292(5519): 1115-1118. |
| 3 | Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: Metabolism of nutrients and other food components. European Journal of Nutrition, 2018, 57(1): 1-24. |
| 4 | Rastelli M, Cani P D, Knauf C. The gut microbiome influences host endocrine functions. Endocrine Reviews, 2019, 40(5): 1271-1284. |
| 5 | Stefania D S, Elisabetta C, Mauro M, et al. Nutritional keys for intestinal barrier modulation. Frontiers in Immunology, 2015, 6: 612. |
| 6 | Long J H, Wang B W, Kong M, et al. Effects of vitamin B12 supplemental level on growth performance, intestinal development and microflora structure in cecum of Wulong geese aged from 5 to 15 weeks. Chinese Journal of Animal Nutrition, 2018, 30(10): 3930-3940. |
| 龙建华, 王宝维, 孔敏, 等. 饲粮中维生素B12添加水平对5~15周龄五龙鹅生长性能、肠道发育和盲肠菌群结构的影响. 动物营养学报, 2018, 30(10): 3930-3940. | |
| 7 | Wang B W, Wang X, Ge W H, et al. Effects of vitamin B2 on growth performance, serum hormone contents and intestinal tissue structure of Wulong geese aged from 5 to 16 weeks. Chinese Journal of Animal Nutrition, 2014, 26(3): 637-645. |
| 王宝维, 王鑫, 葛文华, 等. 维生素B2对5~16周龄五龙鹅生长性能、血清激素含量和肠道组织结构的影响. 动物营养学报, 2014, 26(3): 637-645. | |
| 8 | Halsted C H. The small intestine in vitamin B12 and folate deficiency. Nutrition Reviews, 1975, 33(2): 33-37. |
| 9 | Wang L, Shah A M, Liu Y, et al. Relationship between true digestibility of dietary phosphorus and gastrointestinal bacteria of goats. PLoS One, 2020, 15(5): e0225018. |
| 10 | Jiang S, Huo D, You Z, et al. The distal intestinal microbiome of hybrids of Hainan black goats and Saanen goats. PLoS One, 2020, 15(1): e0228496. |
| 11 | Clemmons B A, Voy B H, Myer P R. Altering the gut microbiome of cattle: Considerations of host-microbiome interactions for persistent microbiome manipulation. Microbial Ecology, 2019, 77(2): 523-536. |
| 12 | Hoover W H. Digestion and absorption in the hindgut of ruminants. Journal of Animal Science, 1978, 46(6): 1789-1799. |
| 13 | Ley R E, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science, 2008, 320(5883): 1647-1651. |
| 14 | Seshadri R, Leahy S C, Attwood G T, et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 collection. Nature Biotechnology, 2018, 36(4): 359-367. |
| 15 | Sasson G, Kruger Ben-shabat S, Seroussi E, et al. Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow’s capacity to harvest energy from its feed. mBio, 2017, 8(4): e00703-17. |
| 16 | Edwin E E, Hebert C N, Jackman R, et al. Thiamine requirement of young ruminants. Journal of Agricultural Science, 1976, 87(3): 679-688. |
| 17 | Burkholder P R, Mcveigh I. Synthesis of vitamins by intestinal bacteria. Proceedings of the National Academy of Sciences of the United States of America, 1942, 28(7): 285-289. |
| 18 | Yao Z H, Mei W Q, Feng Y Y, et al. Effects of dietary supplementation with different doses of vitamin B-complex on the growth performance and microflora composition in goats. Animal Husbandry & Veterinary Medicine, 2020, 52(7): 47-53. |
| 姚志浩, 梅文晴, 冯宇妍, 等. 复合维生素B对山羊生长性能及肠道微生物区系的影响. 畜牧与兽医, 2020, 52(7): 47-53. | |
| 19 | Caporaso J G, Lauber C L, Walters W A, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(Supple 1): 4516-4522. |
| 20 | Youssef N, Sheik C S, Krumholz L R, et al. Comparison of species richness estimates obtained using nearly complete fragments and simulated pyrosequencing-generated fragments in 16S rRNA gene-based environmental surveys. Applied and Environmental Microbiology, 2009, 75(16): 5227-5236. |
| 21 | Haas B J, Gevers D, Earl A M, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Research, 2011, 21(3): 494-504. |
| 22 | Edgar R C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 2013, 10(10): 996. |
| 23 | Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Research, 2013, 41(D1): 590-596. |
| 24 | O'hara E, Neves A L A, Song Y, et al. The role of the gut microbiome in cattle production and health: Driver or passenger? Annual Review of Animal Biosciences, 2020, 8: 199-220. |
| 25 | Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(44): 15718-15723. |
| 26 | Hooper L V. Bacterial contributions to mammalian gut development. Trends in Microbiology, 2004, 12(3): 129-134. |
| 27 | Hooper L V, Wong M H, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science, 2001, 291(5505): 881-884. |
| 28 | Waters J L, Ley R E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biology, 2019, 17(1): 83. |
| 29 | Goodrich J K, Waters J L, Poole A C, et al. Human genetics shape the gut microbiome. Cell, 2014, 159(4): 789-799. |
| 30 | Wang C, Li W, Wang H, et al. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiology, 2019, 19(1): 246. |
| 31 | Egerton S, Donoso F, Fitzgerald P, et al. Investigating the potential of fish oil as a nutraceutical in an animal model of early life stress. Nutritional Neuroscience, 2020(155): 1-23. |
| 32 | Lemaire M, Dou S, Cahu A, et al. Addition of dairy lipids and probiotic Lactobacillus fermentum in infant formula programs gut microbiota and entero-insular axis in adult minipigs. Scientific Reports, 2018, 8(1): 1-16. |
| 33 | Özdemir F, Arslan S. Molecular characterization and toxin profiles of Bacillus spp. isolated from retail fish and ground beef. Journal of Food Science, 2019, 84(3): 548-556. |
| 34 | Caro-Quintero A, Ritalahti K M, Cusick K D, et al. The chimeric genome of Sphaerochaeta: Nonspiral spirochetes that break with the prevalent dogma in spirochete biology. mBio, 2012, 3(3): e00025-12. |
| 35 | Caspary W F. Physiology and pathophysiology of intestinal absorption. American Journal of Clinical Nutrition, 1992, 55(Supple 1): 299-308. |
| 36 | Abbas B, Hayes T L, Wilson D J, et al. Internal structure of the intestinal villus: Morphological and morphometric observations at different levels of the mouse villus. Journal of Anatomy, 1989, 162: 263-273. |
| 37 | Sparks M I, Collins E N. The role of vitamin B1 in tonus of the large intestine. American Journal of Digestive Diseases, 1935, 2(10): 618-620. |
| 38 | Yates C A, Evans G S, Powers H J. Riboflavin deficiency: Early effects on post-weaning development of the duodenum in rats. British Journal of Nutrition, 2001, 86(5): 593-599. |
| 39 | Berkes J, Viswanathan V K, Savkovic S D, et al. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut, 2003, 52(3): 439-451. |
| 40 | Zeisel M B, Dhawan P, BaumertT F. Tight junction proteins in gastrointestinal and liver disease. Gut, 2019, 68(3): 547-561. |
| 41 | Zhang Q, Li Q, Wang C, et al. Enteropathogenic Escherichia coli changes distribution of occludin and ZO-1 in tight junction membrane microdomains in vivo. Microbial Pathogenesis, 2010, 48(1): 28-34. |
| 42 | Wang W J, Sun D Y, Sun X F, et al. Research progress on intestinal barrier function damage and bacterial translocation. Feed Research, 2012(7): 38-40. |
| 王文娟, 孙冬岩, 孙笑非, 等. 肠道屏障功能损伤与细菌易位研究进展. 饲料研究, 2012(7): 38-40. | |
| 43 | Lu N, Wang L, Cao H, et al. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. Journal of Immunology, 2014, 192(3): 1013-1023. |
| [1] | 李俊年, 康绍华, 杨冬梅, 何纤, 李双, 陶双伦. 葛藤草粉替代苜蓿草粉对波杂山羊血清生化指标、养分表观消化率和生产性能的影响[J]. 草业学报, 2021, 30(8): 146-153. |
| [2] | 孙旺斌, 付琪, 薛瑞林, 王伟萍, 张骞, 冯平. 不同枣粉添加水平对陕北白绒山羊屠宰性能和肉品质的影响[J]. 草业学报, 2021, 30(7): 111-121. |
| [3] | 黄丽琴, 李松桥, 袁振中, 唐晶, 闫景彩, 唐启源. 全株水稻与平菇菌糠共发酵料对浏阳黑山羊屠宰性能、肉品质和器官指数的影响[J]. 草业学报, 2021, 30(6): 133-140. |
| [4] | 霍俊宏, 詹康, 黄秋生, 钟小军, 占今舜, 严学兵. 不同精粗比日粮对山羊生产性能、血清生化指标和瘤胃发酵的影响[J]. 草业学报, 2021, 30(6): 151-161. |
| [5] | 熊忙利, 吴旭锦, 朱小甫, 张文娟. 不同苹果渣水平对关中奶山羊泌乳性能、养分表观消化率、血清生化指标及瘤胃液pH值的影响[J]. 草业学报, 2021, 30(3): 81-88. |
| [6] | 王继卿, 沈继源, 刘秀, 李少斌, 罗玉柱, 赵孟丽, 郝志云, 柯娜, 宋宜泽, 乔莉蓉. 子午岭黑山羊与辽宁绒山羊产肉性能、肉品质、肌肉营养成分和脂肪酸含量比较[J]. 草业学报, 2021, 30(2): 166-177. |
| [7] | 占今舜, 霍俊宏, 胡耀, 钟小军, 武艳平. 不同精粗比全混合日粮对努比亚山羊肉品质、血清指标和器官发育的影响[J]. 草业学报, 2020, 29(10): 139-148. |
| [8] | 赵娜, 杨雪海, 陈芳, 郭万正, 李晓峰, 魏金涛, 陈明新, 周广生, 傅廷栋, 谭志平. 青贮饲用油菜对育肥期山羊瘤胃发酵参数及微生物多样性的影响[J]. 草业学报, 2019, 28(9): 146-154. |
| [9] | 姜辉, 雷赵民, 焦婷, 刘婷, 王建福, 李冲, 唐德富, 张建强. 日粮中添加牛至油对河西绒山羊育肥性能的影响研究[J]. 草业学报, 2018, 27(11): 142-149. |
| [10] | 罗燕文, 田平, 华灿枫, 陶诗煜, 倪迎冬. 不同高精料耐受时间对泌乳期山羊肝脏糖脂代谢的影响[J]. 草业学报, 2018, 27(1): 177-186. |
| [11] | 李弘伟, 刘军花, 霍文捷, 朱伟云, 毛胜勇. 高精料日粮对山羊瘤胃和盲肠发酵及生物胺生成与吸收的影响研究[J]. 草业学报, 2017, 26(6): 210-216. |
| [12] | 田平, 孙利利, 董海波, 田靖, 端木永前, 陶诗煜, 倪迎冬. 饲喂高精料日粮对泌乳期山羊泌乳性能及抗氧化能力的影响[J]. 草业学报, 2017, 26(4): 99-105. |
| [13] | 刘军花, 朱伟云, 毛胜勇. 高谷物日粮促进山羊瘤胃上皮单羧酸转运蛋白1及钠钾ATP酶mRNA的表达[J]. 草业学报, 2017, 26(2): 95-101. |
| [14] | 耿雅丽, 田平, 罗燕文, 华灿枫, 陶诗煜, 田靖, 倪迎冬. 高精料对泌乳奶山羊瘤胃上皮氧化应激和胆固醇代谢的影响[J]. 草业学报, 2017, 26(11): 94-103. |
| [15] | 史传奇, 刘玫, 王臣. 东北豆科山羊豆族4属植物叶形态特征及数量分类学研究[J]. 草业学报, 2015, 24(5): 182-189. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||