

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2025, Vol. 34 ›› Issue (7): 219-234.DOI: 10.11686/cyxb2024344
• 综合评述 • 上一篇
收稿日期:2024-09-03
修回日期:2024-10-21
出版日期:2025-07-20
发布日期:2025-05-12
通讯作者:
康俊梅
作者简介:E-mail: kangjunmei@caas.cn基金资助:
Xue-qian JIANG( ), Qing-chuan YANG, Jun-mei KANG(
), Qing-chuan YANG, Jun-mei KANG( )
)
Received:2024-09-03
Revised:2024-10-21
Online:2025-07-20
Published:2025-05-12
Contact:
Jun-mei KANG
摘要:
紫花苜蓿是种植面积最广的多年生豆科饲草,由于其产量高、品质优良而被誉为“牧草之王”。干旱胁迫会对紫花苜蓿生长发育的各个阶段造成严重影响,导致产量损失。干旱胁迫对紫花苜蓿发芽率、分枝形成、茎伸长、叶片发育、根系发育等造成影响,可导致饲草产量减少70%以上。利用分子育种加速培育耐旱性苜蓿新品种是应对干旱胁迫的有效策略。然而紫花苜蓿抗旱性相关的遗传研究基础相对薄弱。前期研究主要集中于转基因和同源克隆。随着紫花苜蓿基因组的发布和测序技术的发展,全基因组关联分析和以转录组测序为代表的组学技术在紫花苜蓿抗旱相关基因的鉴定和抗旱遗传机制的解析中发挥了越来越重要的作用。本研究全面总结了干旱胁迫对紫花苜蓿产量的影响,并概述了近年来在紫花苜蓿抗旱性遗传研究领域取得的进展,旨在为紫花苜蓿抗旱育种提供参考依据。
蒋学乾, 杨青川, 康俊梅. 紫花苜蓿在干旱胁迫下的产量损失与抗旱性遗传研究进展[J]. 草业学报, 2025, 34(7): 219-234.
Xue-qian JIANG, Qing-chuan YANG, Jun-mei KANG. Research progress on yield loss under drought stress and drought resistance genetics of alfalfa (Medicago sativa)[J]. Acta Prataculturae Sinica, 2025, 34(7): 219-234.
材料数量 Material number | 环境 Environment | 发育阶段 Developmental stages | 干旱相关表型 Drought-related phenotype | 产量损失 Yield loss | 参考文献 References |
|---|---|---|---|---|---|
3份材料 3 materials | 花盆,温室 Pots, greenhouse | 苗期 Seedling stage | 总干重和成活率下降,但根冠比增加。 Total dry weight and survival rates decreased, but root-shoot ratio increased. | 陇中苜蓿、陇东苜蓿和甘农3号生物量分别减少25.31%, 40.35%和 69.59%。Biomass of M. sativa cv. Longzhong, Longdong and Gannong No. 3 decreased by 25.31%, 40.35% and 69.59%, respectively. | [ |
8份材料 8 materials | 花盆,温室Pots, greenhouse | 营养生长期 Vegetative stage | 鲜重、干重减少,根茎干重比增加。 Fresh weight, dry weight decreased, but root-shoot dry weight ratio increased. | 产量损失为55%~75%。The yield decreased from 55% to 75%. | [ |
10份材料 10 materials | 温室 Greenhouse | 苗期 Seedling stage | 茎和根的鲜重和干重、根和茎长减少,根茎长度比值增加。Stem and root fresh and dry weight, root and stem length decreased, but root-stem length ratio increased. | - | [ |
3份材料 3 materials | 花盆,温室 Pots, greenhouse | 苗期 Seedling stage | 根系总长度、根系总表面积、根系平均直径、根体积和根尖数、根系干重、根直径降低。Total root length, total root surface area, average root diameter, root volume and number of root tips, root dry weight and root diameter decreased. | - | [ |
8份材料 8 materials | 温室 Greenhouse | 发芽期和苗期 Germination and seedling stages | 发芽阶段:发芽率、胚芽和胚根的鲜重和干重降低;幼苗阶段:根长、茎长、叶面积、叶片数、根和茎干重降低,根茎长度比增加。Germination stage: germination rate, fresh weight and dry weight of plumules and radicles decreased; Seedling stage: root length, stem length, leaf area, number of leaves, root and stem dry weight decreased, but root-stem length ratio increased. | - | [ |
10份材料 10 materials | 田间 Field | 营养生长期 Vegetative stage | 产量损失。 Yield loss. | 产量损失为22%~52%。The yield decreased from 22% to 52%. | [ |
| 198份材料198 materials | 花盆,温室 Pots, greenhouse | 营养生长期 Vegetative stage | 鲜重、干重减少。 Fresh weight, dry weight decreased. | 平均鲜重减少了61.9%,平均干重减少38.1%。The average fresh weight decreased by 61.9%, and the average dry weight decreased by 38.1%. | [ |
| 198份材料198 materials | 田间 Field | 营养生长期 Vegetative stage | 鲜重减少。 Fresh weight decreased. | 3次刈割的苜蓿产量损失分别为:37.4%、3.5%和71.3%。The alfalfa yield decreased by 37.4%, 3.5% and 71.3% in three successive harvest, respectively. | [ |
5份材料 5 materials | 田间 Field | 营养生长期 Vegetative stage | 鲜重、干重、株高、单位面积分枝数、叶面积指数、节间长度和节间数减少,但叶/茎增加。Fresh forage yield, dry forage yield, plant height, stem number/unit area, leaf area index, internode length and internode number decreased, but leaf-stem ratio increased. | 较轻度干旱胁迫,重度干旱胁迫下平均产量损失为37%。Compared with mild drought stress, the average yield loss under severe drought stress was 37%. | [ |
6份材料 6 materials | 培养皿,温室Petri dish, greenhouse | 发芽期 Germination stage | 发芽率、胚根、胚芽长度、种子活力指数降低。Germination rate, radicle and plumule length, and seed vitality index decreased. | - | [ |
11份材料 11 materials | 温室 Greenhouse | 苗期Seedling stage | 生物量降低、根茎比增加。Biomass decreased, but root to stem (R/S) ratio increased. | - | [ |
1份材料 1 material | 花盆,温室 Pots, greenhouse | 营养生长期 Vegetative stage | 全株生物量、叶片数、茎伸长率和枝条相对生长速率、枝条/根减少。Whole plant biomass, leaf number, stem elongation rate and shoot relative growth rate, and shoot-root ratio decreased. | 干重减少了 51%。The dry weight decreased by 51%. | [ |
5份材料 5 materials | 温室 Greenhouse | 营养生长期 Vegetative stage | 干重、存活率、分枝数、根生物量减少。The shoot dry weight, survival rate, number of branches, and root biomass decreased. | 第二次刈割时干重减少了27.3%,第3次刈割时减少了96.5%。The dry weight decreased by 27.3% in second harvest, and 96.5% in third harvest. | [ |
4份材料 4 materials | 温室 Greenhouse | 营养生长期 Vegetative stage | 干重减少。 Dry weight decreased. | 干重减少22.48%~34.45%。The dry weight decreased from 22.48% to 34.45%. | [ |
18份材料 18 materials | 田间 Field | 营养生长期 Vegetative stage | 茎干重、总生物量减少。 Shoot dry matter, total biomass decreased. | 平均茎干重减少28.5%,总干物质减少36.5%。The average stem dry weight decreased by 28.5%, and the total dry matter decreased by 36.5%. | [ |
16份材料 16 materials | 田间 Field | 营养生长期 Vegetative stage | 鲜重、干重减少、叶茎比降低。Fresh and dry weight, leaf-stem ratio decreased. | 干重减少13.8%~46.2%。The dry weight decreased by 13.8%-46.2%. | [ |
表 1 干旱胁迫对紫花苜蓿形态、产量的影响
Table 1 The impact of drought stress on alfalfa morphology and yield
材料数量 Material number | 环境 Environment | 发育阶段 Developmental stages | 干旱相关表型 Drought-related phenotype | 产量损失 Yield loss | 参考文献 References |
|---|---|---|---|---|---|
3份材料 3 materials | 花盆,温室 Pots, greenhouse | 苗期 Seedling stage | 总干重和成活率下降,但根冠比增加。 Total dry weight and survival rates decreased, but root-shoot ratio increased. | 陇中苜蓿、陇东苜蓿和甘农3号生物量分别减少25.31%, 40.35%和 69.59%。Biomass of M. sativa cv. Longzhong, Longdong and Gannong No. 3 decreased by 25.31%, 40.35% and 69.59%, respectively. | [ |
8份材料 8 materials | 花盆,温室Pots, greenhouse | 营养生长期 Vegetative stage | 鲜重、干重减少,根茎干重比增加。 Fresh weight, dry weight decreased, but root-shoot dry weight ratio increased. | 产量损失为55%~75%。The yield decreased from 55% to 75%. | [ |
10份材料 10 materials | 温室 Greenhouse | 苗期 Seedling stage | 茎和根的鲜重和干重、根和茎长减少,根茎长度比值增加。Stem and root fresh and dry weight, root and stem length decreased, but root-stem length ratio increased. | - | [ |
3份材料 3 materials | 花盆,温室 Pots, greenhouse | 苗期 Seedling stage | 根系总长度、根系总表面积、根系平均直径、根体积和根尖数、根系干重、根直径降低。Total root length, total root surface area, average root diameter, root volume and number of root tips, root dry weight and root diameter decreased. | - | [ |
8份材料 8 materials | 温室 Greenhouse | 发芽期和苗期 Germination and seedling stages | 发芽阶段:发芽率、胚芽和胚根的鲜重和干重降低;幼苗阶段:根长、茎长、叶面积、叶片数、根和茎干重降低,根茎长度比增加。Germination stage: germination rate, fresh weight and dry weight of plumules and radicles decreased; Seedling stage: root length, stem length, leaf area, number of leaves, root and stem dry weight decreased, but root-stem length ratio increased. | - | [ |
10份材料 10 materials | 田间 Field | 营养生长期 Vegetative stage | 产量损失。 Yield loss. | 产量损失为22%~52%。The yield decreased from 22% to 52%. | [ |
| 198份材料198 materials | 花盆,温室 Pots, greenhouse | 营养生长期 Vegetative stage | 鲜重、干重减少。 Fresh weight, dry weight decreased. | 平均鲜重减少了61.9%,平均干重减少38.1%。The average fresh weight decreased by 61.9%, and the average dry weight decreased by 38.1%. | [ |
| 198份材料198 materials | 田间 Field | 营养生长期 Vegetative stage | 鲜重减少。 Fresh weight decreased. | 3次刈割的苜蓿产量损失分别为:37.4%、3.5%和71.3%。The alfalfa yield decreased by 37.4%, 3.5% and 71.3% in three successive harvest, respectively. | [ |
5份材料 5 materials | 田间 Field | 营养生长期 Vegetative stage | 鲜重、干重、株高、单位面积分枝数、叶面积指数、节间长度和节间数减少,但叶/茎增加。Fresh forage yield, dry forage yield, plant height, stem number/unit area, leaf area index, internode length and internode number decreased, but leaf-stem ratio increased. | 较轻度干旱胁迫,重度干旱胁迫下平均产量损失为37%。Compared with mild drought stress, the average yield loss under severe drought stress was 37%. | [ |
6份材料 6 materials | 培养皿,温室Petri dish, greenhouse | 发芽期 Germination stage | 发芽率、胚根、胚芽长度、种子活力指数降低。Germination rate, radicle and plumule length, and seed vitality index decreased. | - | [ |
11份材料 11 materials | 温室 Greenhouse | 苗期Seedling stage | 生物量降低、根茎比增加。Biomass decreased, but root to stem (R/S) ratio increased. | - | [ |
1份材料 1 material | 花盆,温室 Pots, greenhouse | 营养生长期 Vegetative stage | 全株生物量、叶片数、茎伸长率和枝条相对生长速率、枝条/根减少。Whole plant biomass, leaf number, stem elongation rate and shoot relative growth rate, and shoot-root ratio decreased. | 干重减少了 51%。The dry weight decreased by 51%. | [ |
5份材料 5 materials | 温室 Greenhouse | 营养生长期 Vegetative stage | 干重、存活率、分枝数、根生物量减少。The shoot dry weight, survival rate, number of branches, and root biomass decreased. | 第二次刈割时干重减少了27.3%,第3次刈割时减少了96.5%。The dry weight decreased by 27.3% in second harvest, and 96.5% in third harvest. | [ |
4份材料 4 materials | 温室 Greenhouse | 营养生长期 Vegetative stage | 干重减少。 Dry weight decreased. | 干重减少22.48%~34.45%。The dry weight decreased from 22.48% to 34.45%. | [ |
18份材料 18 materials | 田间 Field | 营养生长期 Vegetative stage | 茎干重、总生物量减少。 Shoot dry matter, total biomass decreased. | 平均茎干重减少28.5%,总干物质减少36.5%。The average stem dry weight decreased by 28.5%, and the total dry matter decreased by 36.5%. | [ |
16份材料 16 materials | 田间 Field | 营养生长期 Vegetative stage | 鲜重、干重减少、叶茎比降低。Fresh and dry weight, leaf-stem ratio decreased. | 干重减少13.8%~46.2%。The dry weight decreased by 13.8%-46.2%. | [ |
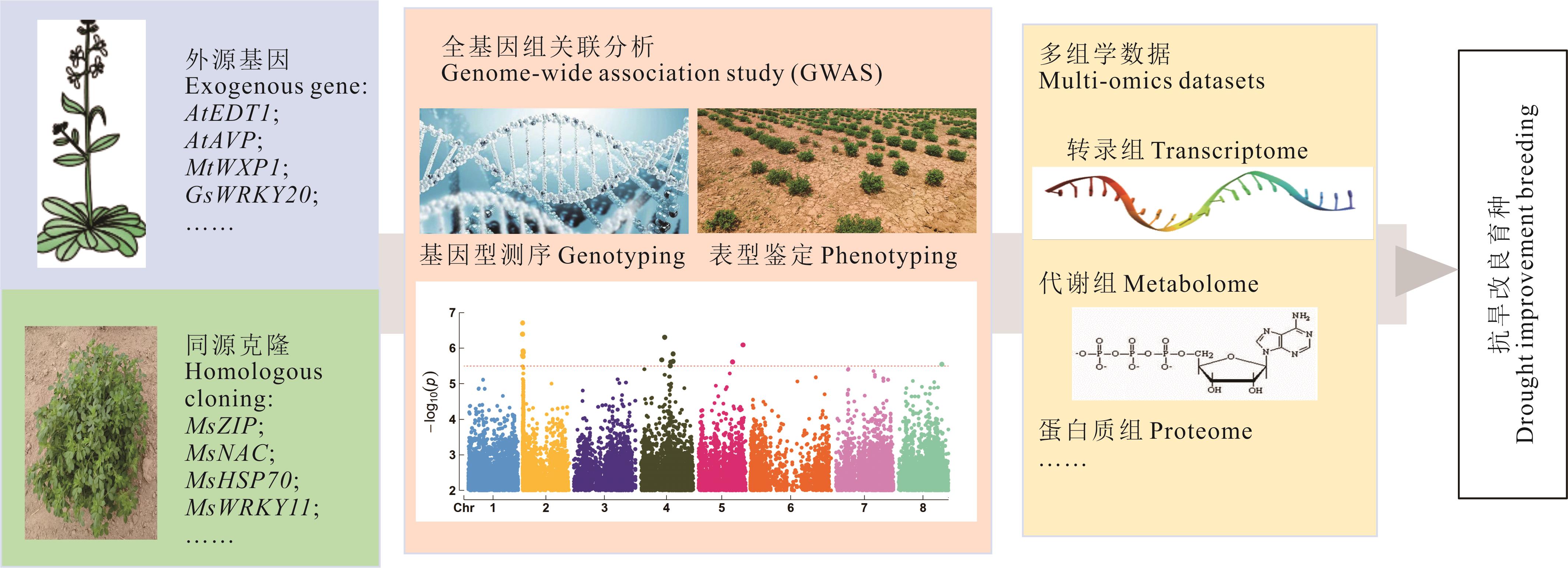
图1 紫花苜蓿抗旱相关基因研究进展由于紫花苜蓿遗传背景复杂,前期的研究多通过在紫花苜蓿中导入外源基因或同源克隆抗旱相关基因以提高其抗旱性。随着测序技术的发展,全基因组关联分析和多组学数据,特别是转录组在紫花苜蓿抗旱相关基因的定位和功能研究中发挥着越来越重要的作用。Due to the complex genetic background of alfalfa, earlier research mainly focused on introducing exogenous genes or homologous cloning of drought-resistant genes to enhance its drought resistance. With the advancement of sequencing technology, genome-wide association analyses and multi-omics data, especially transcriptomics, are playing an increasingly important role in locating and studying the functions of drought-resistant genes in alfalfa.
Fig.1 Research progress on genes related to drought resistance of alfalfa
群体大小 Population size (No.) | 基因型测序 Genotyping | 环境 Environment | 表型 Phenotype | 主要结果 Main results | 参考文献 Reference |
|---|---|---|---|---|---|
| 200 | GBS | 田间 Field | 一年3次刈割的生物量Biomass of three harvests in one year | 干旱条件下定位到28个与生物量相关的单核苷酸多态性(single nucleotide polymorphism, SNP)标记。28 SNP markers were associated with biomass under drought stress. | [ |
| 198 | GBS | 温室 Greenhouse | 耐旱指数和叶片相对含水量Drought resistance index (DRI) and leaf relative water content (RWC) | 在DRI和RWC中分别鉴定了19和15个SNP。Nineteen and fifteen SNP associated with DRI and RWC, respectively. | [ |
| 198 | GBS | 田间 Field | 26个品质相关性状26 forage quality traits | 131个SNP与所有水分亏缺处理中的多个性状相关。131 SNP associated with multiple traits in all the water deficit treatments. | [ |
| 109 | RNA-seq | 温室 Greenhouse | 株高、地上生物量和7个叶绿素荧光参数的抗旱系数Drought-resistance coefficients for plant height, above-ground biomass, and seven chlorophyll fluorescence parameters | 在9个性状中共定位到21个显著SNP;验证了候选基因MsMYBH可增强苜蓿的抗旱性。A total of 21 significant SNPs were identified in nine traits; MsMYBH was confirmed to enhance alfalfa drought resistance. | [ |
表2 紫花苜蓿抗旱性全基因组关联分析研究汇总
Table 2 Summary of genome-wide association studies researches on drought resistance of alfalfa
群体大小 Population size (No.) | 基因型测序 Genotyping | 环境 Environment | 表型 Phenotype | 主要结果 Main results | 参考文献 Reference |
|---|---|---|---|---|---|
| 200 | GBS | 田间 Field | 一年3次刈割的生物量Biomass of three harvests in one year | 干旱条件下定位到28个与生物量相关的单核苷酸多态性(single nucleotide polymorphism, SNP)标记。28 SNP markers were associated with biomass under drought stress. | [ |
| 198 | GBS | 温室 Greenhouse | 耐旱指数和叶片相对含水量Drought resistance index (DRI) and leaf relative water content (RWC) | 在DRI和RWC中分别鉴定了19和15个SNP。Nineteen and fifteen SNP associated with DRI and RWC, respectively. | [ |
| 198 | GBS | 田间 Field | 26个品质相关性状26 forage quality traits | 131个SNP与所有水分亏缺处理中的多个性状相关。131 SNP associated with multiple traits in all the water deficit treatments. | [ |
| 109 | RNA-seq | 温室 Greenhouse | 株高、地上生物量和7个叶绿素荧光参数的抗旱系数Drought-resistance coefficients for plant height, above-ground biomass, and seven chlorophyll fluorescence parameters | 在9个性状中共定位到21个显著SNP;验证了候选基因MsMYBH可增强苜蓿的抗旱性。A total of 21 significant SNPs were identified in nine traits; MsMYBH was confirmed to enhance alfalfa drought resistance. | [ |
基因 Genes | 描述 Description | 基因功能 Gene function | 参考文献Reference |
|---|---|---|---|
| GsZFP1 | 编码Cys2/His2型锌指蛋白Encodes a Cys2/His2-type zinc-finger protein | 过表达增强了紫花苜蓿的耐旱性。Overexpression enhanced drought resistance of alfalfa. | [ |
| GsWRKY20 | WRAKY转录因子WRKY-type transcription factor | 更厚的角质层Thicker cuticular layer | [ |
| miR156/SPL13+DFR/WD40-1 | miRNA,SPL转录因子miRNA, squamosa promoter binding protein-like (SPL) transcription factors | 中等水平的miR156表达可抑制SPL13并增加WD40-1表达,以微调花青素生物合成的DFR表达,并调节紫花苜蓿的各种发育、生理和生化过程,从而提高抗旱能力。Moderate levels of miR156 expression can inhibit SPL13 and increase WD40-1 expression, to fine-tune the expression of DFR involved in anthocyanin biosynthesis, and regulate various developmental, physiological, and biochemical processes in alfalfa, thereby enhancing drought resistance. | [ |
| MsMYBH | MYB-like转录因子MYB-like transcription factor | 保持水平衡、高光合作用效率以及清除过量的H2O2。Maintaining water balance, high photosynthetic efficiency, and scavenging excess H2O2. | [ |
| AtAVP1 | 液泡 H+-焦磷酸酶 (H+-PPase) Vacuolar H+-pyrophosphatase (H+-PPase) | 叶片和根系中积累更多的Na+、K+和Ca2+。Accumulation of more Na+, K+ and Ca2+ in leaves and roots. | [ |
| ZxABCG11 | 编码ABC转运蛋白Encodes an ATP binding cassette (ABC) transporter | 更高的蜡晶体密度和更厚的叶片角质层,从而提高转基因苜蓿的保水能力和光合作用能力。Higher wax crystal density and thicker leaf cuticular layer, thereby enhancing water retention and photosynthetic capacity of transgenic alfalfa. | [ |
| AtEDT1 | 同源域亮氨酸拉链转录因子Homodomain-leucine zipper transcription factor | 降低气孔密度,增加根系发育,同时膜透性和丙二醛含量降低,但可溶性糖和脯氨酸含量较高。Reduced stomatal density, increased root development, while membrane permeability and malondialdehyde content decreased, but soluble sugar and proline content were higher. | [ |
| MtWXP1 | AP2结构域的转录因子基因AP2 domain-containing transcription factor gene | 增加角质层蜡堆积,增强耐旱性。Increased accumulation of cuticular wax, enhancing drought resistance. | [ |
| EsMcsu1 | 编码一种钼辅因子硫化酶Encoding a molybdenum cofactor sulfurase | 促进脱落酸生物合成,提高抗旱性。Promotes abscisic acid biosynthesis, enhancing drought resistance. | [ |
| CsLEA | 晚期胚胎发生丰富蛋白Late embryogenesis abundant (LEA) proteins | 较高的相对含水量和减少的膜损伤。Higher relative water content and reduced membrane damage. | [ |
| AgcodA | 来自土壤细菌 Arthrobacter globiformis 的 codA 基因,编码胆碱氧化酶A codA gene from the soil bacterium (Arthrobacter globiformis), encoding choline oxidase | 保持较高的相对含水量和增加甘氨酸甜菜碱和脯氨酸含量。Maintaining high relative water contents and increased levels of glycinebetaine and proline. | [ |
| MsZIP | bZIP转录因子bZIP transcription factor | 增加丙二醛含量、相对含水量、可溶性糖含量、可溶性蛋白含量和脯氨酸含量。Increased malondialdehyde content, relative water content, soluble sugar content, soluble protein content, and proline content. | [ |
| MsNAC | NAC转录因子NAC transcription factor | - | [ |
| MsHSP17.7 | 编码热休克蛋白Encodes a small heat shock protein | 增加根长。Increased root length. | [ |
| MsZEP | 编码玉米黄质环氧化酶Encodes zeaxanthin epoxidase | 影响各种生理途径、ABA水平和胁迫响应基因表达。Affects various physiological pathways, ABA levels, and stress-responsive gene expression. | [ |
| MsHSP70 | 编码热休克蛋白Encodes heat shock proteins | 相对含水量、脯氨酸含量、超氧化物歧化酶活性升高,丙二醛含量降低。Increased relative water content, proline content, and superoxide dismutase activity, while malondialdehyde content decreased. | [ |
| MsLEA4-4 | 晚期胚胎发生丰富蛋白Late embryogenesis abundant (LEA) proteins | 更多的侧根和更高的叶绿素含量,可溶性糖水平和多种抗氧化酶活性升高,而脯氨酸和丙二醛水平显著降低。More lateral roots and higher chlorophyll content, increased soluble sugar levels and various antioxidant enzyme activities, while proline and malondialdehyde levels significantly decreased. | [ |
| MsCML46 | 编码钙调蛋白样蛋白Encodes calmodulin-like protein | MsCML46 结合游离Ca2+以促进信号转导并维持较高的 K+/Na+,保护细胞内稳态。MsCML46 binds free Ca2+ to promote signal transduction and maintain a higher K+/Na+, protecting cellular homeostasis. | [ |
| MsVDAC | 编码电压依赖性阴离子选择性通道(VDAC)蛋白 Encodes voltage-dependent anion-selective channel (VDAC) protein | 渗透稳态和胁迫响应基因表达。 Osmotic homeostasis and stress-responsive gene expression. | [ |
| MsWRKY11 | WRKY转录因子WRKY transcription factor | 通过调控木质素生物合成和紫花苜蓿气孔开闭调控耐旱性。Enhances drought resistance by regulating lignin biosynthesis and alfalfa stomatal opening and closing. | [ |
| MsDHN1-MsPIP2;1-MsmMYB | MsDHN1(脱水蛋白)、 MsPIP2;1(水通道蛋白),MsmMYB(一种膜锚定的 MYB 转录因子 ) MsDHN1 (dehydrin) and MsPIP2;1 (aquaporin), MsmMYB (a membrane-anchored MYB transcriptional factor MsmMYB) | 缺水导致MsPIP2;1磷酸化,mMYB (mMYB△83)的C端易位并与MsDHN1相互作用,并促进mMYB△83响应缺水的转录活性。mMYB和mMYBD83的过表达下调MsCESA3的表达,但通过直接结合其启动子上调MsCESA7的表达。Dehydration leads to phosphorylation of MsPIP2;1, C-terminal translocation of mMYB (mMYB△83) and interaction with MsDHN1, promoting the transcriptional activity of mMYB△83 in response to dehydration. Overexpression of mMYB and mMYBD83 downregulates the expression of MsCESA3, but upregulates the expression of MsCESA7 by directly binding to its promoter. | [ |
| MsSPL8 | SPL转录因子Squamosa promoter binding protein-like (SPL) transcription factors | 表达下调的植株延缓枯萎并快速恢复; MsSPL8突变体在耐缺水能力方面有所提高。Down-regulation delayed wilting and recovered quickly; MsSPL8 mutants displayed improvements in their ability to withstand water-deficit. | [ |
| MsDIUP1 | 干旱诱导的未知蛋白 1(它缺乏任何可靠的保守结构域)Drought-induced unknown protein 1 (it lacked any confidently conserved domains) | 参与胁迫信号传导、抗氧化防御和渗透调节的基因存在差异反应。Differential responses of genes involved in stress signal transduction, antioxidant defense, and osmotic regulation. | [ |
| MsNTF2L | 核转运因子2-like Nuclear transport factor 2-like | 通过调节叶片失水(通过调节气孔和蜡沉积)、抗氧化防御和光合作用调控苜蓿耐旱性。Regulates drought resistance by modulating leaf dehydration (through stomatal and wax deposition), antioxidant defense, and photosynthesis in alfalfa. | [ |
| miR156/SPL9 | miRNA | 调节花青素的生物合成。Regulating anthocyanin biosynthesis. | [ |
| TPS1-TPS2 | 酵母海藻糖-6-磷酸合酶 (TPS1) 和海藻糖-6-磷酸磷酸酶 (TPS2) 基因Yeast trehalose-6-phosphate synthase (TPS1) and trehalose-6-phosphate phosphatase (TPS2) genes | 海藻糖积累。Accumulation of trehalose. | [ |
| MfLEA3 | 晚期胚胎发生丰富蛋白 Late embryogenesis abundant (LEA) proteins | 转基因植物中积累ROS减少。 Reduced accumulation of ROS in transgenic plants. | [ |
| AtNDPK2 | 拟南芥核苷二磷酸激酶 2 Arabidopsis nucleoside diphosphate kinase 2 | 水分流失率降低,细胞膜损伤降低。 Water loss rate and cell membrane damage were decreased. | [ |
| AtABF3 | 脱落酸(ABA)响应元件结合因子3 ABA-responsive element-binding factor 3 | 蒸腾速率降低,活性氧含量降低。A reduced transpiration rate and lower reactive oxygen species contents. | [ |
| IbOr | 甘薯橙基因The sweetpotato orange gene | 总类胡萝卜素水平更高,细胞膜损伤更少。Higher total carotenoid levels and lower cell membrane damage. | [ |
| co-expression of bar+CsALDH genes | 氧化应答和抗除草剂基因An oxidative responsive gene (CsALDH) and herbicide resistance gene (bar) | Na+含量降低,K+含量升高;离子毒性降低,渗透调节维持;相对含水量升高,光系统变化减少,膜损伤减少。Lower Na+ and higher K+ content; Reduction of ion toxicity and maintenance of osmotic adjustment; Higher relative water content level, fewer changes in the photosystem, decreased membrane injury. | [ |
| MsTMT | 编码γ-生育酚甲基转移酶Encodes γ-tocopherol methyltransferase | 减轻氧化损伤,积累更多渗透解离物质,提高水分利用效率。Alleviated oxidative damage, accumulation of more osmolytic substances and improved water use efficiency. | [ |
表3 紫花苜蓿耐旱性遗传研究进展
Table 3 Advances in genetic research on drought tolerance in alfalfa
基因 Genes | 描述 Description | 基因功能 Gene function | 参考文献Reference |
|---|---|---|---|
| GsZFP1 | 编码Cys2/His2型锌指蛋白Encodes a Cys2/His2-type zinc-finger protein | 过表达增强了紫花苜蓿的耐旱性。Overexpression enhanced drought resistance of alfalfa. | [ |
| GsWRKY20 | WRAKY转录因子WRKY-type transcription factor | 更厚的角质层Thicker cuticular layer | [ |
| miR156/SPL13+DFR/WD40-1 | miRNA,SPL转录因子miRNA, squamosa promoter binding protein-like (SPL) transcription factors | 中等水平的miR156表达可抑制SPL13并增加WD40-1表达,以微调花青素生物合成的DFR表达,并调节紫花苜蓿的各种发育、生理和生化过程,从而提高抗旱能力。Moderate levels of miR156 expression can inhibit SPL13 and increase WD40-1 expression, to fine-tune the expression of DFR involved in anthocyanin biosynthesis, and regulate various developmental, physiological, and biochemical processes in alfalfa, thereby enhancing drought resistance. | [ |
| MsMYBH | MYB-like转录因子MYB-like transcription factor | 保持水平衡、高光合作用效率以及清除过量的H2O2。Maintaining water balance, high photosynthetic efficiency, and scavenging excess H2O2. | [ |
| AtAVP1 | 液泡 H+-焦磷酸酶 (H+-PPase) Vacuolar H+-pyrophosphatase (H+-PPase) | 叶片和根系中积累更多的Na+、K+和Ca2+。Accumulation of more Na+, K+ and Ca2+ in leaves and roots. | [ |
| ZxABCG11 | 编码ABC转运蛋白Encodes an ATP binding cassette (ABC) transporter | 更高的蜡晶体密度和更厚的叶片角质层,从而提高转基因苜蓿的保水能力和光合作用能力。Higher wax crystal density and thicker leaf cuticular layer, thereby enhancing water retention and photosynthetic capacity of transgenic alfalfa. | [ |
| AtEDT1 | 同源域亮氨酸拉链转录因子Homodomain-leucine zipper transcription factor | 降低气孔密度,增加根系发育,同时膜透性和丙二醛含量降低,但可溶性糖和脯氨酸含量较高。Reduced stomatal density, increased root development, while membrane permeability and malondialdehyde content decreased, but soluble sugar and proline content were higher. | [ |
| MtWXP1 | AP2结构域的转录因子基因AP2 domain-containing transcription factor gene | 增加角质层蜡堆积,增强耐旱性。Increased accumulation of cuticular wax, enhancing drought resistance. | [ |
| EsMcsu1 | 编码一种钼辅因子硫化酶Encoding a molybdenum cofactor sulfurase | 促进脱落酸生物合成,提高抗旱性。Promotes abscisic acid biosynthesis, enhancing drought resistance. | [ |
| CsLEA | 晚期胚胎发生丰富蛋白Late embryogenesis abundant (LEA) proteins | 较高的相对含水量和减少的膜损伤。Higher relative water content and reduced membrane damage. | [ |
| AgcodA | 来自土壤细菌 Arthrobacter globiformis 的 codA 基因,编码胆碱氧化酶A codA gene from the soil bacterium (Arthrobacter globiformis), encoding choline oxidase | 保持较高的相对含水量和增加甘氨酸甜菜碱和脯氨酸含量。Maintaining high relative water contents and increased levels of glycinebetaine and proline. | [ |
| MsZIP | bZIP转录因子bZIP transcription factor | 增加丙二醛含量、相对含水量、可溶性糖含量、可溶性蛋白含量和脯氨酸含量。Increased malondialdehyde content, relative water content, soluble sugar content, soluble protein content, and proline content. | [ |
| MsNAC | NAC转录因子NAC transcription factor | - | [ |
| MsHSP17.7 | 编码热休克蛋白Encodes a small heat shock protein | 增加根长。Increased root length. | [ |
| MsZEP | 编码玉米黄质环氧化酶Encodes zeaxanthin epoxidase | 影响各种生理途径、ABA水平和胁迫响应基因表达。Affects various physiological pathways, ABA levels, and stress-responsive gene expression. | [ |
| MsHSP70 | 编码热休克蛋白Encodes heat shock proteins | 相对含水量、脯氨酸含量、超氧化物歧化酶活性升高,丙二醛含量降低。Increased relative water content, proline content, and superoxide dismutase activity, while malondialdehyde content decreased. | [ |
| MsLEA4-4 | 晚期胚胎发生丰富蛋白Late embryogenesis abundant (LEA) proteins | 更多的侧根和更高的叶绿素含量,可溶性糖水平和多种抗氧化酶活性升高,而脯氨酸和丙二醛水平显著降低。More lateral roots and higher chlorophyll content, increased soluble sugar levels and various antioxidant enzyme activities, while proline and malondialdehyde levels significantly decreased. | [ |
| MsCML46 | 编码钙调蛋白样蛋白Encodes calmodulin-like protein | MsCML46 结合游离Ca2+以促进信号转导并维持较高的 K+/Na+,保护细胞内稳态。MsCML46 binds free Ca2+ to promote signal transduction and maintain a higher K+/Na+, protecting cellular homeostasis. | [ |
| MsVDAC | 编码电压依赖性阴离子选择性通道(VDAC)蛋白 Encodes voltage-dependent anion-selective channel (VDAC) protein | 渗透稳态和胁迫响应基因表达。 Osmotic homeostasis and stress-responsive gene expression. | [ |
| MsWRKY11 | WRKY转录因子WRKY transcription factor | 通过调控木质素生物合成和紫花苜蓿气孔开闭调控耐旱性。Enhances drought resistance by regulating lignin biosynthesis and alfalfa stomatal opening and closing. | [ |
| MsDHN1-MsPIP2;1-MsmMYB | MsDHN1(脱水蛋白)、 MsPIP2;1(水通道蛋白),MsmMYB(一种膜锚定的 MYB 转录因子 ) MsDHN1 (dehydrin) and MsPIP2;1 (aquaporin), MsmMYB (a membrane-anchored MYB transcriptional factor MsmMYB) | 缺水导致MsPIP2;1磷酸化,mMYB (mMYB△83)的C端易位并与MsDHN1相互作用,并促进mMYB△83响应缺水的转录活性。mMYB和mMYBD83的过表达下调MsCESA3的表达,但通过直接结合其启动子上调MsCESA7的表达。Dehydration leads to phosphorylation of MsPIP2;1, C-terminal translocation of mMYB (mMYB△83) and interaction with MsDHN1, promoting the transcriptional activity of mMYB△83 in response to dehydration. Overexpression of mMYB and mMYBD83 downregulates the expression of MsCESA3, but upregulates the expression of MsCESA7 by directly binding to its promoter. | [ |
| MsSPL8 | SPL转录因子Squamosa promoter binding protein-like (SPL) transcription factors | 表达下调的植株延缓枯萎并快速恢复; MsSPL8突变体在耐缺水能力方面有所提高。Down-regulation delayed wilting and recovered quickly; MsSPL8 mutants displayed improvements in their ability to withstand water-deficit. | [ |
| MsDIUP1 | 干旱诱导的未知蛋白 1(它缺乏任何可靠的保守结构域)Drought-induced unknown protein 1 (it lacked any confidently conserved domains) | 参与胁迫信号传导、抗氧化防御和渗透调节的基因存在差异反应。Differential responses of genes involved in stress signal transduction, antioxidant defense, and osmotic regulation. | [ |
| MsNTF2L | 核转运因子2-like Nuclear transport factor 2-like | 通过调节叶片失水(通过调节气孔和蜡沉积)、抗氧化防御和光合作用调控苜蓿耐旱性。Regulates drought resistance by modulating leaf dehydration (through stomatal and wax deposition), antioxidant defense, and photosynthesis in alfalfa. | [ |
| miR156/SPL9 | miRNA | 调节花青素的生物合成。Regulating anthocyanin biosynthesis. | [ |
| TPS1-TPS2 | 酵母海藻糖-6-磷酸合酶 (TPS1) 和海藻糖-6-磷酸磷酸酶 (TPS2) 基因Yeast trehalose-6-phosphate synthase (TPS1) and trehalose-6-phosphate phosphatase (TPS2) genes | 海藻糖积累。Accumulation of trehalose. | [ |
| MfLEA3 | 晚期胚胎发生丰富蛋白 Late embryogenesis abundant (LEA) proteins | 转基因植物中积累ROS减少。 Reduced accumulation of ROS in transgenic plants. | [ |
| AtNDPK2 | 拟南芥核苷二磷酸激酶 2 Arabidopsis nucleoside diphosphate kinase 2 | 水分流失率降低,细胞膜损伤降低。 Water loss rate and cell membrane damage were decreased. | [ |
| AtABF3 | 脱落酸(ABA)响应元件结合因子3 ABA-responsive element-binding factor 3 | 蒸腾速率降低,活性氧含量降低。A reduced transpiration rate and lower reactive oxygen species contents. | [ |
| IbOr | 甘薯橙基因The sweetpotato orange gene | 总类胡萝卜素水平更高,细胞膜损伤更少。Higher total carotenoid levels and lower cell membrane damage. | [ |
| co-expression of bar+CsALDH genes | 氧化应答和抗除草剂基因An oxidative responsive gene (CsALDH) and herbicide resistance gene (bar) | Na+含量降低,K+含量升高;离子毒性降低,渗透调节维持;相对含水量升高,光系统变化减少,膜损伤减少。Lower Na+ and higher K+ content; Reduction of ion toxicity and maintenance of osmotic adjustment; Higher relative water content level, fewer changes in the photosystem, decreased membrane injury. | [ |
| MsTMT | 编码γ-生育酚甲基转移酶Encodes γ-tocopherol methyltransferase | 减轻氧化损伤,积累更多渗透解离物质,提高水分利用效率。Alleviated oxidative damage, accumulation of more osmolytic substances and improved water use efficiency. | [ |
| 1 | Nadeem M, Li J J, Yahya M, et al. Research progress and perspective on drought stress in legumes: A review. International Journal of Molecular Sciences, 2019, 20(10): 2541. |
| 2 | Inès S, Talbi O, Nasreddine Y, et al. Drought tolerance traits in Medicago species: A review. Arid Land Research Management, 2022, 36(1): 67-83. |
| 3 | Bartels D, Sunkar R. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences, 2005, 24(1): 23-58. |
| 4 | Kim W, Iizumi T, Nishimori M. Global patterns of crop production losses associated with droughts from 1983 to 2009. Journal of Applied Meteorology Climatology, 2019, 58(6): 1233-1244. |
| 5 | Matiu M, Ankerst D P, Menzel A. Interactions between temperature and drought in global and regional crop yield variability during 1961-2014. PLoS One, 2017, 12(5): e0178339. |
| 6 | Gupta A, Rico-Medina A, Caño-Delgado A I. The physiology of plant responses to drought. Science, 2020, 368(6488): 266-269. |
| 7 | Comas L H, Becker S R, Cruz V M V, et al. Root traits contributing to plant productivity under drought. Frontiers in Plant Science, 2013, 4: 442. |
| 8 | Yadav B, Jogawat A, Gnanasekaran P, et al. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene, 2021, 25: 100264. |
| 9 | Li Y P, Ye W, Wang M, et al. Climate change and drought: a risk assessment of crop-yield impacts. Climate Research, 2009, 39(1): 31-46. |
| 10 | Yu L X, Kole C. The alfalfa genome. Cham, Switzerland: Springer, 2021. |
| 11 | Radović J, Sokolović D, Marković J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnology in Animal Husbandry, 2009, 25(5/6): 465-475. |
| 12 | Bora K S, Sharma A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharmaceutical Biology, 2011, 49(2): 211-220. |
| 13 | Li A, Liu A, Du X, et al. A chromosome-scale genome assembly of a diploid alfalfa, the progenitor of autotetraploid alfalfa. Horticulture Research, 2020, 7: 194. |
| 14 | Bai Z, Ma W, Ma L, et al. China’s livestock transition: Driving forces, impacts, and consequences. Science Advances, 2018, 4(7): eaar8534. |
| 15 | Wang Q B, Zou Y. China’s alfalfa market and imports: Development, trends, and potential impacts of the US-China trade dispute and retaliations. Journal of Integrative Agriculture, 2020, 19(4): 1149-1158. |
| 16 | Ashrafi M, Azimi-Moqadam M R, Moradi P, et al. Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant Physiology and Biochemistry, 2018, 132: 391-399. |
| 17 | Kumar S. Biotechnological advancements in alfalfa improvement. Journal of Applied Genetics, 2011, 52(2): 111-124. |
| 18 | Gall H L, Philippe F, Domon J M, et al. Cell wall metabolism in response to abiotic stress. Plants, 2015, 4(1): 112-166. |
| 19 | Zhang C, Shi S, Liu Z, et al. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. Journal of Plant Physiology, 2019, 232: 226-240. |
| 20 | Kapoor D, Bhardwaj S, Landi M, et al. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Applied Sciences, 2020, 10(16): 5692. |
| 21 | Zhang H, Zhu J, Gong Z, et al. Abiotic stress responses in plants. Nature Reviews Genetics, 2022, 23(2): 104-119. |
| 22 | Davis R, Earl H, Timper P. Effect of simultaneous water deficit stress and Meloidogyne incognita infection on cotton yield and fiber quality. Journal of Nematology, 2014, 46(2): 108. |
| 23 | Ranjan A, Sinha R, Singla-Pareek S L, et al. Shaping the root system architecture in plants for adaptation to drought stress. Physiologia Plantarum, 2022, 174(2): e13651. |
| 24 | Maqbool S, Hassan M A, Xia X, et al. Root system architecture in cereals: progress, challenges and perspective. The Plant Journal, 2022, 110(1): 23-42. |
| 25 | Rogers E D, Benfey P N. Regulation of plant root system architecture: implications for crop advancement. Current Opinion in Biotechnology, 2015, 32: 93-98. |
| 26 | Seo D H, Seomun S, Choi Y D, et al. Root development and stress tolerance in rice: the key to improving stress tolerance without yield penalties. International Journal of Molecular Sciences, 2020, 21(5): 1807. |
| 27 | Jeong J S, Kim Y S, Redillas M C, et al. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnology Journal, 2013, 11(1): 101-114. |
| 28 | Castroluna A, Ruiz O, Quiroga A, et al. Effects of salinity and drought stress on germination, biomass and growth in three varieties of Medicago sativa L. Avances en Investigación Agropecuaria, 2014, 18(1): 39-50. |
| 29 | Slama I, Tayachi S, Jdey A, et al. Differential response to water deficit stress in alfalfa (Medicago sativa) cultivars: Growth, water relations, osmolyte accumulation and lipid peroxidation. African Journal of Biotechnology, 2011, 10(72): 16250-16259. |
| 30 | Riasat M, Saed-Mouchehsi A, Jafari A A. Effect of drought stress levels on seedling morpho-physiological traits of alfalfa (Medicago sativa) populations grown in glasshouse. Journal of Rangeland Science, 2020, 10(1): 86-97. |
| 31 | Zhang C M, Shi S L, Liu Z, et al. Effects of drought stress on the root morphology and anatomical structure of alfalfa (Medicago sativa) varieties with differing drought-tolerance. Acta Prataculturae Sinica, 2019, 28(5): 79-89. |
| 张翠梅, 师尚礼, 刘珍,等. 干旱胁迫对不同抗旱性苜蓿品种根系形态及解剖结构的影响. 草业学报, 2019, 28(5): 79-89. | |
| 32 | Lamb J, Barnes D, Henjum K J C S. Gain from two cycles of divergent selection for root morphology in alfalfa. Crop Science, 1999, 39(4): 1026-1035. |
| 33 | Tang L, Cai H, Ji W, et al. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.). Plant Physiology and Biochemistry, 2013, 71: 22-30. |
| 34 | Tang L, Cai H, Zhai H, et al. Overexpression of Glycine soja WRKY20 enhances both drought and salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Cell, Tissue and Organ Culture, 2014, 118: 77-86. |
| 35 | Aung B, Gruber M Y, Amyot L, et al. MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnology Journal, 2015, 13(6): 779-790. |
| 36 | Arshad M, Feyissa B A, Amyot L, et al. MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing SPL13. Plant Science, 2017, 258: 122-136. |
| 37 | Feyissa B A, Arshad M, Gruber M Y, et al. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biology, 2019, 19(1): 1-19. |
| 38 | Safarnejad A J P J B. Morphological and biochemical response to osmotic stress in alfalfa (Medicago sativa L.). Pakistan Journal of Botany, 2008, 40(2): 735-746. |
| 39 | Maghsoodi M, Razmjoo J. Identify physiological markers for drought tolerance in alfalfa. Agronomy Journal, 2015, 107(1): 149-157. |
| 40 | Zhang T, Kesoju S, Greene S L, et al. Genetic diversity and phenotypic variation for drought resistance in alfalfa (Medicago sativa L.) germplasm collected for drought tolerance. Genetic Resources and Crop Evolution, 2018, 65: 471-484. |
| 41 | Yu L X. Identification of single-nucleotide polymorphic loci associated with biomass yield under water deficit in alfalfa (Medicago sativa L.) using genome-wide sequencing and association mapping. Frontiers in Plant Science, 2017, 8: 1152. |
| 42 | Afsharmanesh G. Study of some morphological traits and selection of drought-resistant alfalfa cultivars (Medicago sativa L.) in Jiroft, Iran. Plant Ecophysiology (Jiroft Branch), 2009, 1(3): 109-118. |
| 43 | Hamidi H, Safarnejad A. Effect of drought stress on alfalfa cultivars (Medicago sativa L.) in germination stage. American-Eurasian Journal of Agricultural and Environmental Sciences, 2010, 8(6): 705-709. |
| 44 | Hanson A, Xu L, Johnson P S, et al. Identification and characterization of drought-tolerant alfalfa (Medicago sativa subsp. falcata) germplasm. Proceedings of the South Dakota Academy of Science, 2015, 94: 263-272. |
| 45 | Gorai M, Hachef A, Neffati M. Differential responses in growth and water relationship of Medicago sativa (L.) cv. Gabès and Astragalus gombiformis (Pom.) under water-limited conditions. Emirates Journal of Food and Agriculture, 2010, 7(1): 1-12. |
| 46 | Annicchiarico P, Pecetti L, Tava A. Physiological and morphological traits associated with adaptation of lucerne (Medicago sativa) to severely drought-stressed and to irrigated environments. Annals of Applied Biology, 2013, 162(1): 27-40. |
| 47 | Farissi M, Bouizgaren A, Faghire M, et al. Agrophysiological and biochemical properties associated with adaptation of Medicago sativa populations to water deficit. Turkish Journal of Botany, 2013, 37(6): 1166-1175. |
| 48 | Moghaddam A, Vollmann J, Wanek W, et al. Suitability of drought tolerance indices for selecting alfalfa (Medicago sativa L.) genotypes under organic farming in Austria. Crop Breeding Journal, 2012, 2(2): 79-89. |
| 49 | Benabderrahim M, Hamza H, Haddad M, et al. Assessing the drought tolerance variability in Mediterranean alfalfa (Medicago sativa L.) genotypes under arid conditions. Plant Biosystems, 2013, 149(2): 395-403. |
| 50 | Boe A, Kephart K D, Berdahl J D, et al. Breeding alfalfa for semiarid regions in the northern Great Plains: History and additional genetic evaluations of novel germplasm. Agronomy, 2020, 10(11): 1686. |
| 51 | Shi S, Nan L, Smith K F. The current status, problems, and prospects of alfalfa (Medicago sativa L.) breeding in China. Agronomy, 2017, 7(1): 1. |
| 52 | Yang Q C. Guide for alfalfa planting zone and cultivar. Beijing: China Agricultural University Press, 2012. |
| 杨青川. 苜蓿种植区划及品种指南. 北京: 中国农业大学出版社, 2012. | |
| 53 | Li X, Brummer E C. Applied genetics and genomics in alfalfa breeding. Agronomy, 2012, 2(1): 40-61. |
| 54 | Volenec J, Cunningham S, Haagenson D, et al. Physiological genetics of alfalfa improvement: past failures, future prospects. Field Crops Research, 2002, 75(2/3): 97-110. |
| 55 | Zhang T, Yu L X, Zheng P, et al. Identification of loci associated with drought resistance traits in heterozygous autotetraploid alfalfa (Medicago sativa L.) using genome-wide association studies with genotyping by sequencing. PLoS One, 2015, 10(9): e0138931. |
| 56 | Lin S, Medina C A, Boge B, et al. Identification of genetic loci associated with forage quality in response to water deficit in autotetraploid alfalfa (Medicago sativa L.). BMC Plant Biology, 2020, 20(1): 1-18. |
| 57 | Shi K, Liu J, Liang H, et al. An alfalfa MYB-like transcriptional factor MsMYBH positively regulates alfalfa seedling drought resistance and undergoes MsWAV3-mediated degradation. Journal of Integrative Plant Biology, 2024, 66(4): 683-699. |
| 58 | Liu X P, Hawkins C, Peel M D, et al. Genetic loci associated with salt tolerance in advanced breeding populations of tetraploid alfalfa using genome‐wide association studies. The Plant Genome, 2019, 12(1): 180026. |
| 59 | Long R C, Zhang F, Zhang Z W, et al. Genome assembly of alfalfa cultivar Zhongmu-4 and identification of SNPs associated with agronomic traits. Genomics, Proteomics and Bioinformatics, 2022, 20(1): 14-28. |
| 60 | Chen H, Zeng Y, Yang Y, et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nature Communications, 2020, 11(1): 2494. |
| 61 | Shen C, Du H, Chen Z, et al. The chromosome-level genome sequence of the autotetraploid alfalfa and resequencing of core germplasms provide genomic resources for alfalfa research. Molecular Plant, 2020, 13(9): 1250-1261. |
| 62 | Bao A K, Wang S M, Wu G Q, et al. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Science, 2009, 176(2): 232-240. |
| 63 | Liu L B, Bao A K, Li H J, et al. Overexpression of ZxABCG11 from Zygophyllum xanthoxylum enhances tolerance to drought and heat in alfalfa by increasing cuticular wax deposition. The Crop Journal, 2023, 11(4): 1140-1151. |
| 64 | Zheng G, Fan C, Di S, et al. Over-expression of Arabidopsis EDT1 gene confers drought tolerance in alfalfa (Medicago sativa L.). Frontiers in Plant Science, 2017, 8: 316380. |
| 65 | Zhang J Y, Broeckling C D, Blancaflor E B, et al. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). The Plant Journal, 2005, 42(5): 689-707. |
| 66 | Jiang Q, Zhang J Y, Guo X, et al. Physiological characterization of transgenic alfalfa (Medicago sativa) plants for improved drought tolerance. International Journal of Plant Sciences, 2009, 170(8): 969-978. |
| 67 | Zhou C, Ma Z, Zhu L, et al. Overexpression of EsMcsu1 from the halophytic plant Eutrema salsugineum promotes abscisic acid biosynthesis and increases drought resistance in alfalfa (Medicago sativa L.). Genetics and Molecular Research, 2015, 14: 17204-17218. |
| 68 | Zhang J, Duan Z, Zhang D, et al. Co-transforming bar and CsLEA enhanced tolerance to drought and salt stress in transgenic alfalfa (Medicago sativa L.). Biochemical and Biophysical Research Communications, 2016, 472(1): 75-82. |
| 69 | Li H, Wang Z, Ke Q, et al. Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiology and Biochemistry, 2014, 85: 31-40. |
| 70 | Li Y, Sun Y, Yang Q, et al. Isolation and characterization of a gene from Medicago sativa L., encoding a bZIP transcription factor. Molecular Biology Reports, 2013, 40(2): 1227-1239. |
| 71 | Wang Y X. Characterization of a novel Medicago sativa NAC transcription factor gene involved in response to drought stress. Molecular Biology Reports, 2013, 40(11): 6451-6458. |
| 72 | Li Z Y, Long R C, Zhang T J, et al. Molecular cloning and characterization of the MsHSP17.7 gene from Medicago sativa L. Molecular Biology Reports, 2016, 43(8): 815-826. |
| 73 | Zhang Z, Wang Y, Chang L, et al. MsZEP, a novel zeaxanthin epoxidase gene from alfalfa (Medicago sativa), confers drought and salt tolerance in transgenic tobacco. Plant Cell Reports, 2016, 35(2): 439-453. |
| 74 | Li Z, Long R, Zhang T, et al. Molecular cloning and functional analysis of the drought tolerance gene MsHSP70 from alfalfa (Medicago sativa L.). Journal of Plant Research, 2017, 130(2): 387-396. |
| 75 | Jia H, Wang X, Shi Y, et al. Overexpression of Medicago sativa LEA 4-4 can improve the salt, drought, and oxidation resistance of transgenic Arabidopsis. PLoS One, 2020, 15(6): e0234085. |
| 76 | Du B, Chen N, Song L, et al. Alfalfa (Medicago sativa L.) MsCML46 gene encoding calmodulin-like protein confers tolerance to abiotic stress in tobacco. Plant Cell Reports, 2021, 40(10): 1907-1922. |
| 77 | Yang M, Duan X, Wang Z, et al. Overexpression of a voltage-dependent anion-selective channel (VDAC) protein-encoding gene, MsVDAC, from Medicago sativa confers cold and drought tolerance to transgenic tobacco. Genes, 2021, 12(11): 1706. |
| 78 | Wen W, Wang R, Su L, et al. MsWRKY11, activated by MsWRKY22, functions in drought tolerance and modulates lignin biosynthesis in alfalfa (Medicago sativa L.). Environmental and Experimental Botany, 2021, 184: 104373. |
| 79 | Lv A, Su L, Fan N, et al. The MsDHN-MsPIP2;1-MsmMYB module orchestrates the trade-off between growth and survival of alfalfa in response to drought stress. Plant Biotechnology Journal, 2023, 22(5): 1132-1145. |
| 80 | Gou J, Debnath S, Sun L, et al. From model to crop: functional characterization of SPL8 in M. truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnology Journal, 2018, 16(4): 951-962. |
| 81 | Singer S D, Burton Hughes K, Subedi U, et al. The CRISPR/Cas9-mediated modulation of squamosa promoter-binding protein-like 8 in alfalfa leads to distinct phenotypic outcomes. Frontiers in Plant Science, 2021, 12: 774146. |
| 82 | Luo D, Zhang X, Liu J, et al. Drought-induced unknown protein 1 positively modulates drought tolerance in cultivated alfalfa (Medicago sativa L.). The Crop Journal, 2023, 11(1): 57-70. |
| 83 | Luo D, Liu J, Wu Y, et al. NUCLEAR TRANSPORT FACTOR 2-LIKE improves drought tolerance by modulating leaf water loss in alfalfa (Medicago sativa L.). The Plant Journal, 2022, 112(2): 429-450. |
| 84 | Waheed S, Zeng L. The critical role of miRNAs in regulation of flowering time and flower development. Genes, 2020, 11(3): 319. |
| 85 | Singroha G, Sharma P, Sunkur R. Current status of microRNA-mediated regulation of drought stress responses in cereals. Physiologia Plantarum, 2021, 172(3): 1808-1821. |
| 86 | Li Y, Wan L, Bi S, et al. Identification of drought-responsive microRNAs from roots and leaves of alfalfa by high-throughput sequencing. Genes, 2017, 8(4): 119. |
| 87 | Arshad M, Gruber M Y, Hannoufa A. Transcriptome analysis of microRNA156 overexpression alfalfa roots under drought stress. Scientific Reports, 2018, 8(1): 9363. |
| 88 | Gao R, Austin R S, Amyot L, et al. Comparative transcriptome investigation of global gene expression changes caused by miR156 overexpression in Medicago sativa. BMC Genomics, 2016, 17(1): 658. |
| 89 | Hanly A, Karagiannis J, Lu Q S M, et al. Characterization of the role of SPL9 in drought stress tolerance in Medicago sativa. International Journal of Molecular Sciences, 2020, 21(17): 6003. |
| 90 | Suárez R, Calderón C, Iturriaga G. Enhanced tolerance to multiple abiotic stresses in transgenic alfalfa accumulating trehalose. Crop Science, 2009, 49(5): 1791-1799. |
| 91 | Shi H, He X, Zhao Y, et al. Constitutive expression of a group 3 LEA protein from Medicago falcata (MfLEA3) increases cold and drought tolerance in transgenic tobacco. Plant Cell Reports, 2020, 39(7): 851-860. |
| 92 | Wang Z, Li H, Ke Q, et al. Transgenic alfalfa plants expressing AtNDPK2 exhibit increased growth and tolerance to abiotic stresses. Plant Physiology and Biochemistry, 2014, 84: 67-77. |
| 93 | Wang Z, Su G, Li M, et al. Overexpressing Arabidopsis ABF3 increases tolerance to multiple abiotic stresses and reduces leaf size in alfalfa. Plant Physiology and Biochemistry, 2016, 109: 199-208. |
| 94 | Wang Z, Ke Q, Kim M D, et al. Transgenic alfalfa plants expressing the sweetpotato orange gene exhibit enhanced abiotic stress tolerance. PLoS One, 2015, 10(5): e0126050. |
| 95 | Duan Z, Zhang D, Zhang J, et al. Co-transforming bar and CsALDH genes enhanced resistance to herbicide and drought and salt stress in transgenic alfalfa (Medicago sativa L.). Frontiers in Plant Science, 2015, 6: 1115. |
| 96 | Ma J, Qiu D, Gao H, et al. Over-expression of a γ-tocopherol methyltransferase gene in vitamin E pathway confers PEG-simulated drought tolerance in alfalfa. BMC Plant Biology, 2020, 20(1): 226. |
| 97 | Naish M, Alonge M, Wlodzimierz P, et al. The genetic and epigenetic landscape of the Arabidopsis centromeres. Science, 2021, 374(6569): eabi7489. |
| 98 | Song J M, Xie W Z, Wang S, et al. Two gap-free reference genomes and a global view of the centromere architecture in rice. Molecular Plant, 2021, 14(10): 1757-1767. |
| 99 | Chen J, Wang Z, Tan K, et al. A complete telomere-to-telomere assembly of the maize genome. Nature Genetics, 2023, 55(7): 1221-1231. |
| 100 | Schreiber M, Jayakodi M, Stein N, et al. Plant pangenomes for crop improvement, biodiversity and evolution. Nature Reviews Genetics, 2024, 25(8): 563-577. |
| 101 | Zhou Y, Zhang Z, Bao Z, et al. Graph pangenome captures missing heritability and empowers tomato breeding. Nature, 2022, 606(7914): 527-534. |
| 102 | Guo J, Cao K, Deng C, et al. An integrated peach genome structural variation map uncovers genes associated with fruit traits. Genome Biology, 2020, 21(1): 258. |
| 103 | Yang L, Yang Y, Huang L, et al. From single- to multi-omics: future research trends in medicinal plants. Briefings in Bioinformatics, 2023, 24(1): bbac485. |
| 104 | Wang M, Wang Y, Li X, et al. Integration of metabolomics and transcriptomics reveals the regulation mechanism of the phenylpropanoid biosynthesis pathway in insect resistance traits in Solanum habrochaites. Horticulture Research, 2024, 11(2): uhad277. |
| 105 | Kuang L, Yan T, Gao F, et al. Multi-omics analysis reveals differential molecular responses to cadmium toxicity in rice root tip and mature zone. Journal of Hazardous Materials, 2024, 462: 132758. |
| 106 | Gao R, Feyissa B A, Croft M, et al. Gene editing by CRISPR/Cas9 in the obligatory outcrossing Medicago sativa. Planta, 2018, 247(4): 1043-1050. |
| 107 | Zhao H, Zhao S, Cao Y, et al. Development of a single transcript CRISPR/Cas9 toolkit for efficient genome editing in autotetraploid alfalfa. The Crop Journal, 2024, 12(3): 788-795. |
| [1] | 赵媛媛, 蒲小剑, 徐成体, 王伟, 傅云洁. 蒺藜苜蓿MtBMI1基因克隆及抗旱性分析[J]. 草业学报, 2025, 34(6): 139-153. |
| [2] | 温小月, 赵颖, 王宝强, 王贤, 朱晓林, 王义真, 魏小红. 外源NO调控干旱胁迫下紫花苜蓿AP2/ERFs基因的表达分析[J]. 草业学报, 2025, 34(6): 154-167. |
| [3] | 姜沛沛, 郭锦花, 肖慧淑, 彭彦珉, 张军, 田文仲, 吕军杰, 吴金芝, 王贺正, 付国占, 黄明, 李友军. 轮耕模式对旱地玉-麦两熟体系作物产量和品质的影响[J]. 草业学报, 2025, 34(6): 181-192. |
| [4] | 刘启林, 王小军, 王金兰, 刘文辉, 马巧玲, 李建辉, 张生原, 曹文侠, 李文. 氮磷配施对高寒区老芒麦饲草产量的影响[J]. 草业学报, 2025, 34(6): 193-202. |
| [5] | 张英豪, 刘楚波, 周坤, 郭家存, 刘世鹏, 孙娈姿. 果草系统中枣树对不同方位紫花苜蓿和鸭茅生长的影响[J]. 草业学报, 2025, 34(6): 203-212. |
| [6] | 秦文利, 张静, 肖广敏, 崔素倩, 叶建勋, 智健飞, 张立锋, 谢楠, 冯伟, 刘振宇, 潘璇, 代云霞, 刘忠宽. 绿肥部分替代化肥氮对土壤物理性状的影响[J]. 草业学报, 2025, 34(6): 27-45. |
| [7] | 崔灿, 王梦琦, 赵琬璐, 刘新颖, 鉴晶晶, 严俊鑫. 胺鲜酯浸种对NaCl胁迫下紫花苜蓿种子萌发及幼苗生长的影响[J]. 草业学报, 2025, 34(6): 46-58. |
| [8] | 曾燕霞, 陈志龙, 尚继红, 沙晓弟, 吴娟, 陈彩锦. 太空诱变对PEG-6000模拟干旱胁迫下紫花苜蓿材料苗期生长的影响[J]. 草业学报, 2025, 34(6): 59-69. |
| [9] | 刘耀博, 裴渌, 刘琛琢, 李晓霞, 邹博坤. 基于Meta分析中国老芒麦种子产量和产量组分对施肥的响应[J]. 草业学报, 2025, 34(6): 85-98. |
| [10] | 魏孔钦, 张盈盈, 回金峰, 马春晖, 张前兵. 菌磷配施对紫花苜蓿根系非结构碳水化合物及碳氮磷化学计量特征的影响[J]. 草业学报, 2025, 34(5): 40-50. |
| [11] | 周昕越, 王丽萍, 蒋庆雪, 马晓冉, 仪登霞, 王学敏. 紫花苜蓿低温诱导蛋白MsLTI65的分离及其对不同逆境的响应[J]. 草业学报, 2025, 34(5): 89-104. |
| [12] | 罗天蓉, 马健芝, 杜明阳, 多杰措, 熊辉岩, 段瑞君. 紫花苜蓿LACS基因家族成员鉴定及表达分析[J]. 草业学报, 2025, 34(4): 124-136. |
| [13] | 冯雅琪, 陈嘉慧, 张静妮, 隋超, 陈基伟, 刘志鹏, 周强, 刘文献. 基于重测序紫花苜蓿高蛋白、高产关联InDel分子标记开发[J]. 草业学报, 2025, 34(4): 137-149. |
| [14] | 董拓轩, 陈训锋, 梅大海, 郭永莎, 魏旭红, 宋秋艳. 纳米铁与铜对苜蓿壳二孢及其引致春季黑茎病的抑制与防治作用[J]. 草业学报, 2025, 34(4): 201-211. |
| [15] | 王腾飞, 马霞, 刘金龙, 王斌, 张译尹, 李佳旺, 马江萍, 王小兵, 兰剑. 引黄灌区复种饲用燕麦种植模式产量、品质及经济效益分析[J]. 草业学报, 2025, 34(4): 27-37. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||