

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2025, Vol. 34 ›› Issue (1): 161-173.DOI: 10.11686/cyxb2024099
• 研究论文 • 上一篇
贺龙义( ), 谭萌萌, 车海涛, 张红鹰, 朱雨欣, 张彦妮(
), 谭萌萌, 车海涛, 张红鹰, 朱雨欣, 张彦妮( )
)
收稿日期:2024-03-26
修回日期:2024-05-08
出版日期:2025-01-20
发布日期:2024-11-04
通讯作者:
张彦妮
作者简介:E-mail: tdcqtgzy@126.com基金资助:
Long-yi HE( ), Meng-meng TAN, Hai-tao CHE, Hong-ying ZHANG, Yu-xin ZHU, Yan-ni ZHANG(
), Meng-meng TAN, Hai-tao CHE, Hong-ying ZHANG, Yu-xin ZHU, Yan-ni ZHANG( )
)
Received:2024-03-26
Revised:2024-05-08
Online:2025-01-20
Published:2024-11-04
Contact:
Yan-ni ZHANG
摘要:
AP2/ERF转录因子是植物特有的一类转录因子,其中DREB亚家族蛋白被广泛报道可以提高植物对非生物胁迫的抵抗能力。为了开发细叶百合DREB家族的功能基因资源,验证DREB转录因子与耐旱调控相关性,本研究以细叶百合根部cDNA为模板,克隆得到LpDREB9基因,对其进行生物信息学分析、亚细胞定位,并通过该基因转化模式植物烟草,开展LpDREB9转录因子耐旱机制方面的研究。结果表明:LpDREB9基因的开放阅读框(ORF)为462 bp,编码153个氨基酸,蛋白的相对分子量为17.054 kDa,脂肪系数为73.46,pI值为4.89,为不稳定且具亲水性的蛋白。亚细胞定位结果表明LpDREB9蛋白定位于细胞核,同源比对结果表明LpDREB9蛋白与岷江百合的同源基因进化关系最为密切。另外通过对野生烟草种子(WT)和转基因LpDREB9烟草种子、幼苗进行脱落酸(abscisic acid,ABA)和干旱胁迫以及对成苗进行自然干旱胁迫及复水后的表型和生理指标的测定,发现LpDREB9基因增强了转基因烟草的耐旱性,并且随着干旱胁迫时间的增加,LpDREB9转基因烟草中超氧化物歧化酶(superoxide dismutase,SOD)、过氧化物酶(peroxidase,POD)和过氧化氢酶(catalase,CAT)活性、叶绿素以及脯氨酸(proline,Pro)含量明显高于WT(P<0.05),而丙二醛(malondialdehyde,MDA)含量则显著低于WT(P<0.05),表明转基因烟草中的膜脂过氧化反应程度较低,活性氧清除能力相对较高,从而提高了其耐旱性。因此,LpDREB9基因在增强转基因烟草耐旱机制方面具有关键作用,这为进一步从分子水平探究细叶百合的抗逆性奠定了基础。
贺龙义, 谭萌萌, 车海涛, 张红鹰, 朱雨欣, 张彦妮. 细叶百合LpDREB9基因克隆及耐旱性分析[J]. 草业学报, 2025, 34(1): 161-173.
Long-yi HE, Meng-meng TAN, Hai-tao CHE, Hong-ying ZHANG, Yu-xin ZHU, Yan-ni ZHANG. Cloning and analysis of drought tolerance function of the LpDREB9 in Lilium pumilum[J]. Acta Prataculturae Sinica, 2025, 34(1): 161-173.
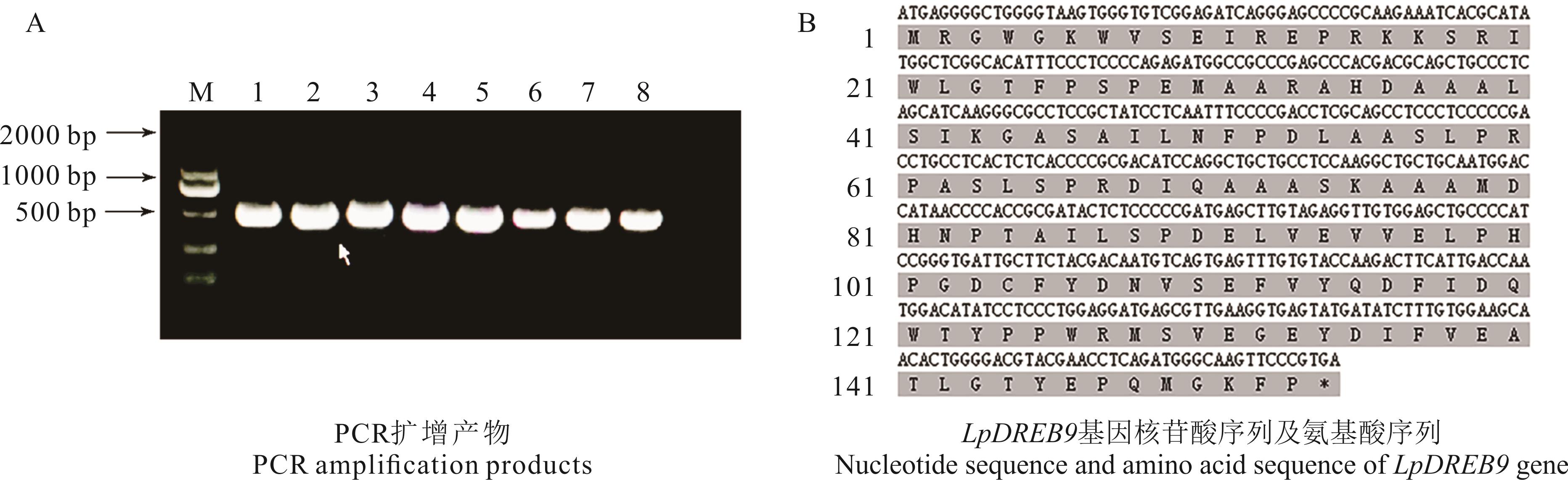
图1 LpDREB9基因克隆M: DL2000 marker; 1~8: LpDREB9基因的PCR扩增产物PCR amplification product of LpDREB9 gene; 灰色标注为氨基酸序列Gray marked as amino acid sequence. 下同The same below.
Fig.1 LpDREB9 gene cloning
基因名称 Gene | 开放阅读框长度 Open reading frame length (bp) | 氨基酸数量 Number of amino acids (aa) | 相对分子量 Relative molecular weight (kDa) | 不稳定系数 Instability coefficient | 脂肪指数 Fat index | 蛋白质等电点 Protein isoelectric point | 亲水指数 Hydropathy index | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| LpDREB9 | 462 | 153 | 17.054 | 43.6 | 73.46 | 4.89 | -0.293 | 细胞核Nucleus |
表1 细叶百合LpDREB9转录因子理化性质分析
Table 1 Physicochemical properties analysis of LpDREB9 transcription factor in L. pumilum
基因名称 Gene | 开放阅读框长度 Open reading frame length (bp) | 氨基酸数量 Number of amino acids (aa) | 相对分子量 Relative molecular weight (kDa) | 不稳定系数 Instability coefficient | 脂肪指数 Fat index | 蛋白质等电点 Protein isoelectric point | 亲水指数 Hydropathy index | 亚细胞定位 Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| LpDREB9 | 462 | 153 | 17.054 | 43.6 | 73.46 | 4.89 | -0.293 | 细胞核Nucleus |

图3 LpDREB9转基因烟草的获得及LpDREB9蛋白亚细胞定位CaMV 35S Pro: 花椰菜花叶病毒的35S 启动子 35S promoter of cauliflower mosaic virus; GFP: 绿色荧光蛋白 Green fluorescent protein; Nos: 终止子Terminator; 菌: 质粒 DNA Plasmid DNA; 水: 无菌蒸馏水Sterile distilled water; CK: 野生型烟草 Wild type (WT) tobacco; 1~7: LpDREB9转基因烟草抗性株系的扩增产物 Amplification products of LpDREB9 transgenic tobacco resistant lines; a: 1/2 MS培养基 1/2 MS medium; b: 筛选培养基(1/2 MS 培养基+25 mg·L-1 Hyg) Screening medium (1/2 MS medium+25 mg·L-1 Hyg). WT: 野生型烟草WT tobacco; Tr 9-2, Tr 9-3, Tr 9-5: 分别为LpDREB9转基因烟草抗性株系 LpDREB9 transgenic tobacco resistant line, respectively.下同The same below.
Fig.3 Acquisition of LpDREB9 transgenic tobacco and subcellular localization of LpDREB9 protein

图4 LpDREB9植株对ABA的敏感性分析不同小写字母表示相同浓度间差异显著(P<0.05,Duncan)。下同。Different lowercase letters indicate significant differences among the same concentration (P<0.05, Duncan). The same below.
Fig.4 Sensitivity analysis of LpDREB9 plants to ABA
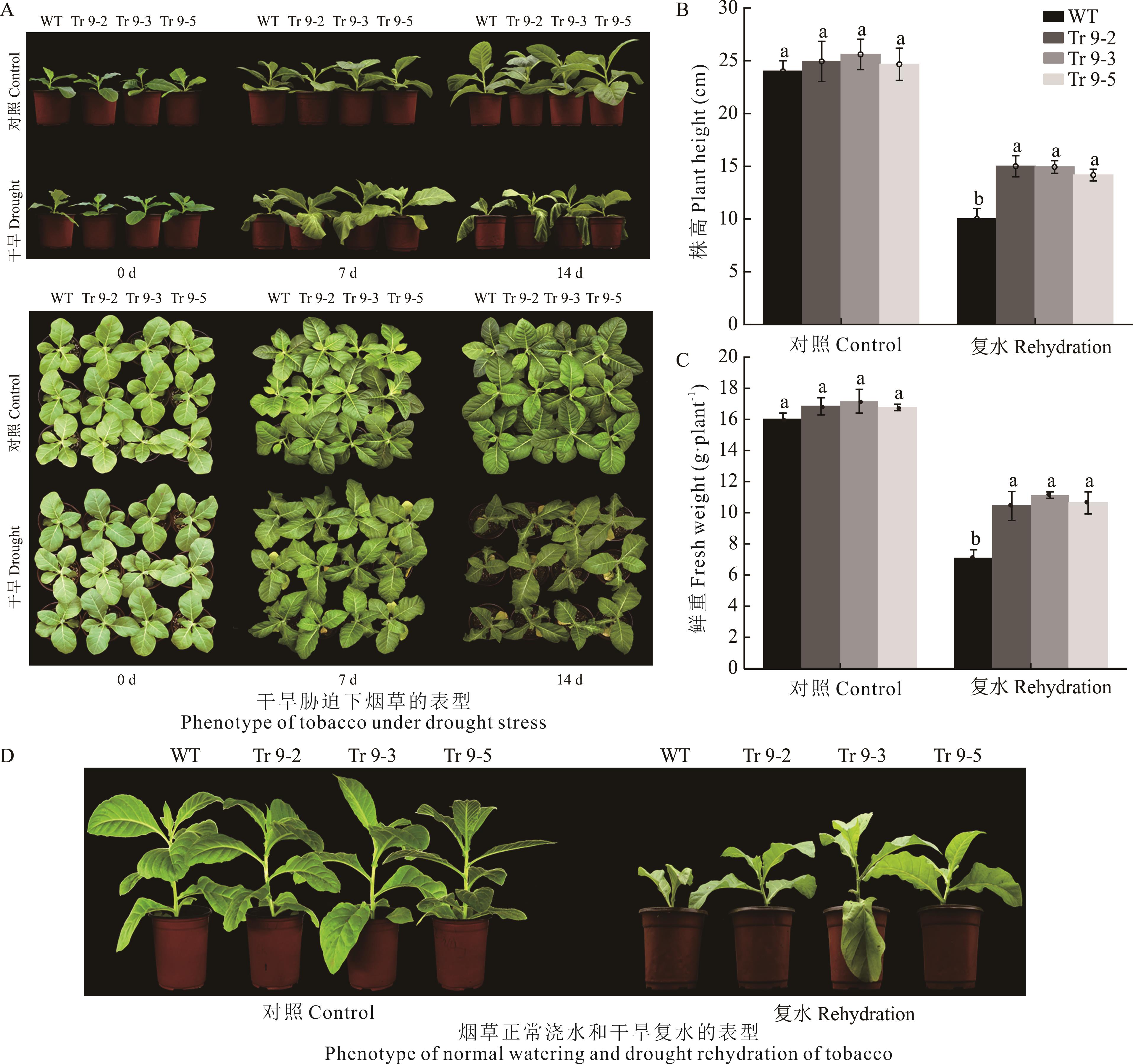
图7 干旱胁迫下烟草成苗的抗性分析不同小写字母表示相同处理间差异显著(P<0.05,Duncan)。Different lowercase letters indicate significant differences among the same treatment (P<0.05, Duncan).
Fig.7 Resistance analysis of tobacco seedling formation under drought stress
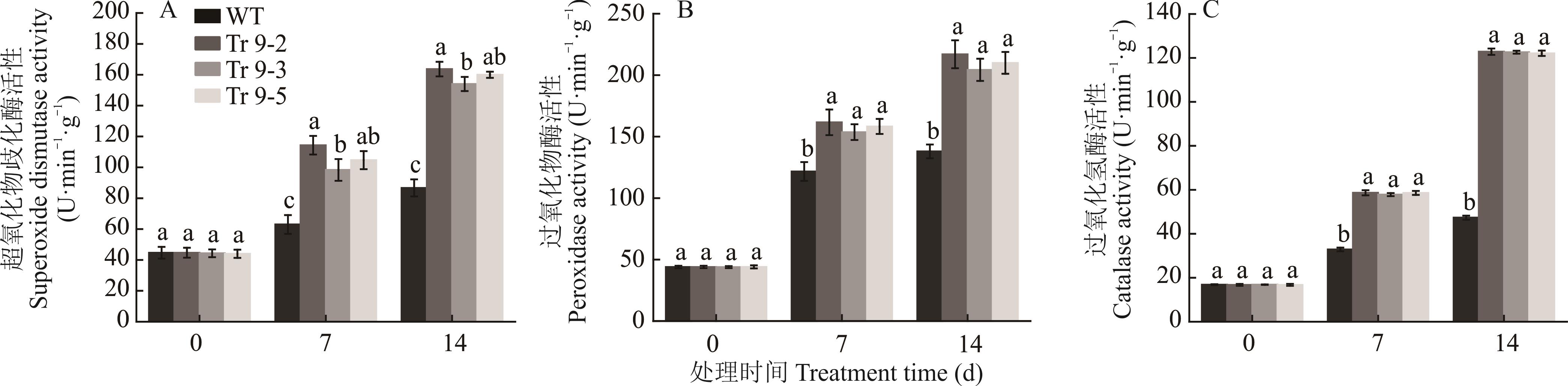
图8 烟草成苗干旱胁迫下不同天数的抗氧化酶活性变化不同小写字母表示相同处理天数间差异显著(P<0.05,Duncan)。下同。Different lowercase letters indicate significant differences among the same treatment day (P<0.05, Duncan). The same below.
Fig.8 The changes of antioxidant enzyme activity in different days under drought stress of tobacco seedlings
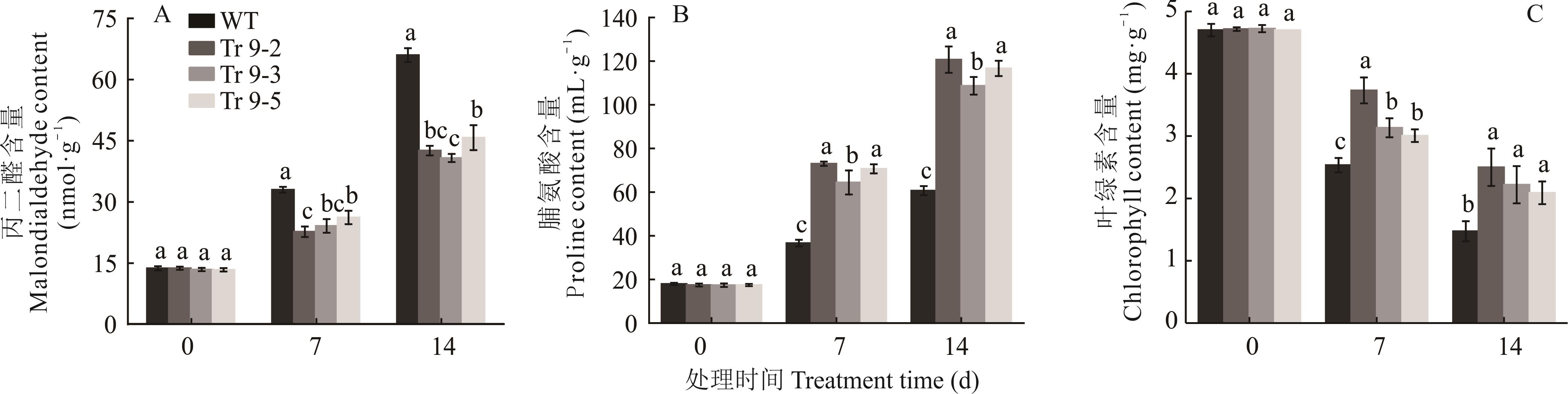
图9 烟草成苗干旱胁迫不同天数下丙二醛、脯氨酸和叶绿素含量变化
Fig.9 Changes of malondialdehyde, proline and chlorophyll content in tobacco seedlings under drought stress in different days
| 1 | Zhu J K. Abiotic stress signaling and responses in plants. Cell, 2016, 167(2): 313-324. |
| 2 | Liang Y, Li X, Yang R, et al. BaDBL1, a unique DREB gene from desiccation tolerant moss Bryum argenteum, confers osmotic and salt stress tolerances in transgenic Arabidopsis. Plant Science, 2021, 313(2021): 111047. |
| 3 | Chai M, Cheng H, Yan M, et al. Identification and expression analysis of the DREB transcription factor family in pineapple [Ananas comosus (L.) Merr.]. PeerJ, 2020, 8(6): e9006. |
| 4 | Yoh S, Qiang L, Dubouzet J G, et al. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochemical and Biophysical Research Communications, 2002, 290(3): 998-1009. |
| 5 | Yang H H, Sun Y G, Wang H X, et al. Genome-wide identification and functional analysis of the ERF2 gene family in response to disease resistance against Stemphylium lycopersici in tomato. BMC Plant Biology, 2021, 21(1): 72. |
| 6 | Most A S, Mohammed N, Kouji S, et al. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant and Cell Physiology, 2021, 52(2): 344-360. |
| 7 | Liu S, Wang X, Wang H, et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genetics, 2013, 9(9): e1003790. |
| 8 | Lucas S, Durmaz E, Akpınar B A, et al. The drought response displayed by a DRE-binding protein from Triticum dicoccoides. Plant Physiology and Biochemistry, 2011, 49(3): 346-351. |
| 9 | Zhao P, Wang D, Wang R, et al. Genome-wide analysis of the potato Hsp20 gene family: identification, genomic organization and expression profiles in response to heat stress. BMC Genomics, 2018, 19(1): 1-13. |
| 10 | Bihani P, Char B, Bhargava S. Transgenic expression of sorghum DREB2 in rice improves tolerance and yield under water limitation. The Journal of Agricultural Science, 2011, 149(1): 95-101. |
| 11 | Rabara R C, Tripathi P, Rushton P J. The potential of transcription factor-based genetic engineering in improving crop tolerance to drought. Omics: A Journal of Integrative Biology, 2014, 18(10): 601-614. |
| 12 | Song Y, Lin W H, Jing Y B, et al. Cloning and expression analysis of Catalase gene in Lilium pumilum. Bulletin of Botanical Research, 2023, 43(5): 756-767. |
| 宋煜, 林文昊, 荆一博, 等. 细叶百合Catalase基因的克隆及表达分析. 植物研究, 2023, 43(5): 756-767. | |
| 13 | Wang Y, Cao S, Guan C, et al. Overexpressing the NAC transcription factor LpNAC13 from Lilium pumilum in tobacco negatively regulates the drought response and positively regulates the salt response. Plant Physiology and Biochemistry, 2020, 149: 96-110. |
| 14 | Guan C J. Cloning of three NAC transcription factors from Lilium pumilum and genetic transformation to tobacco. Harbin: Northeast Forestry University, 2018. |
| 关春景. 细叶百合3个NAC转录因子的克隆及其对烟草的遗传转化. 哈尔滨: 东北林业大学, 2018. | |
| 15 | He H, Zhu G Q, Chen S Y, et al. Cloning of LpPEX7 gene from Lilium pumilum and its expression characteristics under salt stress. Bulletin of Botanical Research, 2020, 40(2): 274-283. |
| 何好, 朱国庆, 陈诗雅, 等. 细叶百合LpPEX7基因克隆及盐胁迫下的表达特性分析. 植物研究, 2020, 40(2): 274-283. | |
| 16 | Tan M M, Sun S Y, Wang J W, et al. Bioinformatics and stress expression analysis of DREB transcription factor in Lilium pumilum. Journal of Northwest Forestry University, 2023, 38(1): 95-101,198. |
| 谭萌萌, 孙绍营, 王静文, 等. 细叶百合DREB转录因子生物信息学及胁迫应答表达分析. 西北林学院学报, 2023, 38(1): 95-101,198. | |
| 17 | Inge C D, Vanessa V, Olivier A V, et al. The membrane-bound NAC transcription factor ANAC013functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. The Plant Cell, 2013, 25(9): 3472-3490. |
| 18 | Sun S Y. Transcriptome analysis of Lilium pumilum under salt stress and verification of salt-tolerance function of LpNAC14. Harbin: Northeast Forestry University, 2022. |
| 孙绍营. 盐胁迫下细叶百合转录组分析及LpNAC14抗盐功能验证. 哈尔滨: 东北林业大学, 2022. | |
| 19 | Wang X K. Principles and techniques of plant physiological and biochemical experiments. Beijing: Higher Education Press, 2006. |
| 王学奎. 植物生理生化实验原理和技术. 北京: 高等教育出版社, 2006. | |
| 20 | Thirumalaikumar V P, Devkar V, Mehterov N, et al. NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnology Journal, 2018, 16(2): 354-366. |
| 21 | Liu Q, Kasuga M, Sakuma Y, et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell, 1998, 10(8): 1391-1406. |
| 22 | Hussain Q, Asim M, Zhang R, et al. Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules, 2021, 11(8): 1159. |
| 23 | Rego D C F T, Santos P M, Cabral B G, et al. Expression of a DREB5-A subgroup transcription factor gene from Ricinus communis (RcDREB1) enhanced growth, drought tolerance and pollen viability in tobacco. Plant Cell, Tissue and Organ Culture (PCTOC), 2021, 146(3): 1-12. |
| 24 | Chen M, Zhao Y J, Zhuo C L, et al. Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnology Journal, 2015, 13(4): 482-491. |
| 25 | Aditi G, Andrés R, Caño-Delgado A I. The physiology of plant responses to drought. Science, 2020, 368(6488): 266-269. |
| 26 | Ron M, Zandalinas S I, Yosef F, et al. Reactive oxygen species signalling in plant stress responses. Nature Reviews Molecular Cell Biology, 2022, 23(10): 663-679. |
| 27 | Lanceras J C. Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiology, 2004, 135(1): 384-399. |
| 28 | Liu B, Cao S J, Wang Y, et al. Overexpression of LpNAC6 gene in Lilium pumilum enhancing salt tolerance in transgenic tobacco. Journal of Beijing Forestry University, 2020, 42(4): 69-79. |
| 刘彬, 曹尚杰, 王营, 等. 过表达细叶百合 LpNAC6 基因增强烟草的耐盐性. 北京林业大学学报, 2020, 42(4): 69-79. | |
| 29 | Chen T, Shabala S, Niu Y. Molecular mechanisms of salinity tolerance in rice. The Crop Journal, 2021, 9(3): 506-520. |
| 30 | Zhu J, Lee B H, Dellinger M, et al. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. The Plant Journal, 2010, 63(1): 128-140. |
| 31 | Zhu Q, Zhang J, Gao X, et al. The Arabidopsis AP2/ERF transcription factor RAP2.6 participates in ABA, salt and osmotic stress responses. Gene, 2010, 457(1): 1-12. |
| 32 | Bapela T, Shimelis H, Tsilo T J, et al. Genetic improvement of wheat for drought tolerance: progress, challenges and opportunities. Plants, 2022, 11(10): 1331. |
| 33 | Shinde S S, Kachare D P, Satbhai R D, et al. Water stress induced proline accumulation and antioxidative enzymes in groundnut ( Arachis hypogaea L.). Legume Research, 2018, 41(1): 67-72. |
| [1] | 魏娜, 敬文茂, 许尔文, 王荣新, 赵晶忠, 马雪娥, 张吉宇, 刘文献. 白花草木樨MaERF058基因耐旱功能验证[J]. 草业学报, 2024, 33(8): 159-169. |
| [2] | 吴毅, 冯雅岚, 王添宁, 琚吉浩, 肖慧淑, 马超, 张均. 小麦及其祖先物种Hsp70基因家族鉴定与表达分析[J]. 草业学报, 2024, 33(7): 53-67. |
| [3] | 张震欢, 姚立蓉, 汪军成, 司二静, 张宏, 杨轲, 马小乐, 孟亚雄, 王化俊, 李葆春. 盐生草AKR基因家族成员的鉴定及根系盐胁迫响应基因HgAKR42639的耐盐分析[J]. 草业学报, 2024, 33(7): 68-83. |
| [4] | 王芳, 张世子, 戴镕徽, 杨丽云, 罗丽娟, 蒋凌雁. 柱花草SgMPK6互作蛋白的筛选与验证[J]. 草业学报, 2024, 33(7): 84-93. |
| [5] | 孔海明, 宋家兴, 杨静, 李倩, 杨培志, 曹玉曼. 紫花苜蓿CAMTA基因家族鉴定及其在非生物胁迫下的表达模式分析[J]. 草业学报, 2024, 33(5): 143-154. |
| [6] | 李显炀, 刘昊, 何飞, 王雪, 李明娜, 龙瑞才, 康俊梅, 杨青川, 陈林. 全基因组水平紫花苜蓿WRKY转录因子家族鉴定与表达模式分析[J]. 草业学报, 2024, 33(4): 154-170. |
| [7] | 慕平, 柴继宽, 苏玮娟, 章海龙, 赵桂琴. 燕麦不同组合正、反交杂种后代的表型及遗传参数分析[J]. 草业学报, 2024, 33(4): 73-86. |
| [8] | 黎泽斌, 邱永争, 刘延杰, 喻金秋, 王柏吉, 刘千宁, 王月, 崔国文. 紫花苜蓿BZR基因家族鉴定及其对非生物胁迫的响应分析[J]. 草业学报, 2024, 33(11): 106-122. |
| [9] | 王凤宇, 梁国玲, 胡泽龙, 刘文辉. 地理因子对青藏高原野生垂穗披碱草表型及种子产量的影响[J]. 草业学报, 2024, 33(11): 198-214. |
| [10] | 史先飞, 高宇, 黄旭升, 周雅莉, 蔡桂萍, 李昕儒, 李润植, 薛金爱. 油莎豆CeWRKY转录因子响应非生物胁迫的功能表征[J]. 草业学报, 2023, 32(8): 186-201. |
| [11] | 张振粉, 黄荣, 李向阳, 姚博, 赵桂琴. 基于Illumina MiSeq高通量测序的燕麦种带细菌多样性及功能分析[J]. 草业学报, 2023, 32(7): 96-108. |
| [12] | 张一龙, 喻启坤, 李雯, 李培英, 孙宗玖. 不同抗旱性狗牙根地上地下表型特征及内源激素对干旱胁迫的响应[J]. 草业学报, 2023, 32(3): 163-178. |
| [13] | 刘福, 陈诚, 张凯旋, 周美亮, 张新全. 日本百脉根LjbHLH34基因克隆及耐旱功能鉴定[J]. 草业学报, 2023, 32(1): 178-191. |
| [14] | 田骄阳, 王秋霞, 郑淑文, 刘文献. 全基因组水平蒺藜苜蓿CPP基因家族的鉴定及表达模式分析[J]. 草业学报, 2022, 31(7): 111-121. |
| [15] | 张勃, 孙淑范. 毛地黄鼠尾草在不同授粉条件下的繁殖成功与花性状选择[J]. 草业学报, 2022, 31(5): 84-91. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||