

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2022, Vol. 31 ›› Issue (10): 75-86.DOI: 10.11686/cyxb2021410
收稿日期:2021-11-09
修回日期:2022-02-08
出版日期:2022-10-20
发布日期:2022-09-14
通讯作者:
刘文辉
作者简介:E-mail: qhliuwenhui@163.com基金资助:
Yu-han WU( ), Wen-hui LIU(
), Wen-hui LIU( ), Kai-qiang LIU, Yong-chao ZHANG
), Kai-qiang LIU, Yong-chao ZHANG
Received:2021-11-09
Revised:2022-02-08
Online:2022-10-20
Published:2022-09-14
Contact:
Wen-hui LIU
摘要:
为探究燕麦幼苗遭受干旱胁迫后叶片光合特性及活性氧清除系统的响应机制,本试验选用青海本地推广品种‘青燕1号’为研究对象,以PEG-6000模拟干旱胁迫环境,在不同干旱浓度(CK、P10和P20)和不同干旱持续时间(4、7、10 d)处理下,研究燕麦幼苗干旱胁迫后对光合作用参数、叶绿素含量、类胡萝卜素含量、超氧阴离子含量、过氧化氢含量及酶类抗氧化剂和非酶抗氧化剂含量的影响,为青藏高原地区抗旱燕麦品种评价提供理论依据。研究表明:1)随着胁迫程度增加叶绿素含量(Chl)显著下降,超氧阴离子(O2-)、过氧化氢(H2O2)含量明显增加,净光合速率(Pn)、蒸腾速率(Tr)、胞间CO2浓度(Ci)、气孔导度(Gs)、最大光化学效率(Fv/Fm)和实际光化学效率(ΦpsⅡ)明显下降,而初始荧光(Fo)和非光化学淬灭(NPQ)显著增加。干旱胁迫能引起植株短时间内水分利用效率增加,但气孔关闭及光反应中心遭到破坏是光合性能减弱的主要原因。2)随胁迫浓度与胁迫时间增加酶类抗氧化剂超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、脱氢抗坏血酸还原酶(DHAR)、谷胱甘肽还原酶(GR)、抗坏血酸过氧化物酶(APX)先升高后降低,谷胱甘肽过氧化物酶(GPX)持续增加,而非酶类抗氧化剂在干旱胁迫下均显著高于对照。轻度胁迫下,‘青燕1号’燕麦主要通过酶类抗氧化剂清除活性氧毒害物质,而在重度胁迫下主要以非酶类抗氧化剂清除系统为主。
吴雨涵, 刘文辉, 刘凯强, 张永超. 干旱胁迫对燕麦幼苗叶片光合特性及活性氧清除系统的影响[J]. 草业学报, 2022, 31(10): 75-86.
Yu-han WU, Wen-hui LIU, Kai-qiang LIU, Yong-chao ZHANG. Effects of drought stress on leaf senescence and the active oxygen scavenging system of oat seedlings[J]. Acta Prataculturae Sinica, 2022, 31(10): 75-86.
| 因素Factors | 叶绿素含量Chl | 类胡萝卜素含量Car | 过氧化氢含量H2O2 | 氧自由基含量O2- |
|---|---|---|---|---|
| 干旱时间Drought time (DT) | 137.592** | 72.904** | 169.602** | 95.278** |
| 干旱程度Drought degree (DD) | 692.148** | 117.385** | 317.876** | 26.757** |
| 干旱时间×干旱程度DT×DD | 27.335** | 66.314** | 100.038** | 32.121** |
表1 干旱胁迫对燕麦叶片生理指标影响的方差分析
Table 1 The variance analysis of effects on oat leaf physiological indicators under drought stress
| 因素Factors | 叶绿素含量Chl | 类胡萝卜素含量Car | 过氧化氢含量H2O2 | 氧自由基含量O2- |
|---|---|---|---|---|
| 干旱时间Drought time (DT) | 137.592** | 72.904** | 169.602** | 95.278** |
| 干旱程度Drought degree (DD) | 692.148** | 117.385** | 317.876** | 26.757** |
| 干旱时间×干旱程度DT×DD | 27.335** | 66.314** | 100.038** | 32.121** |
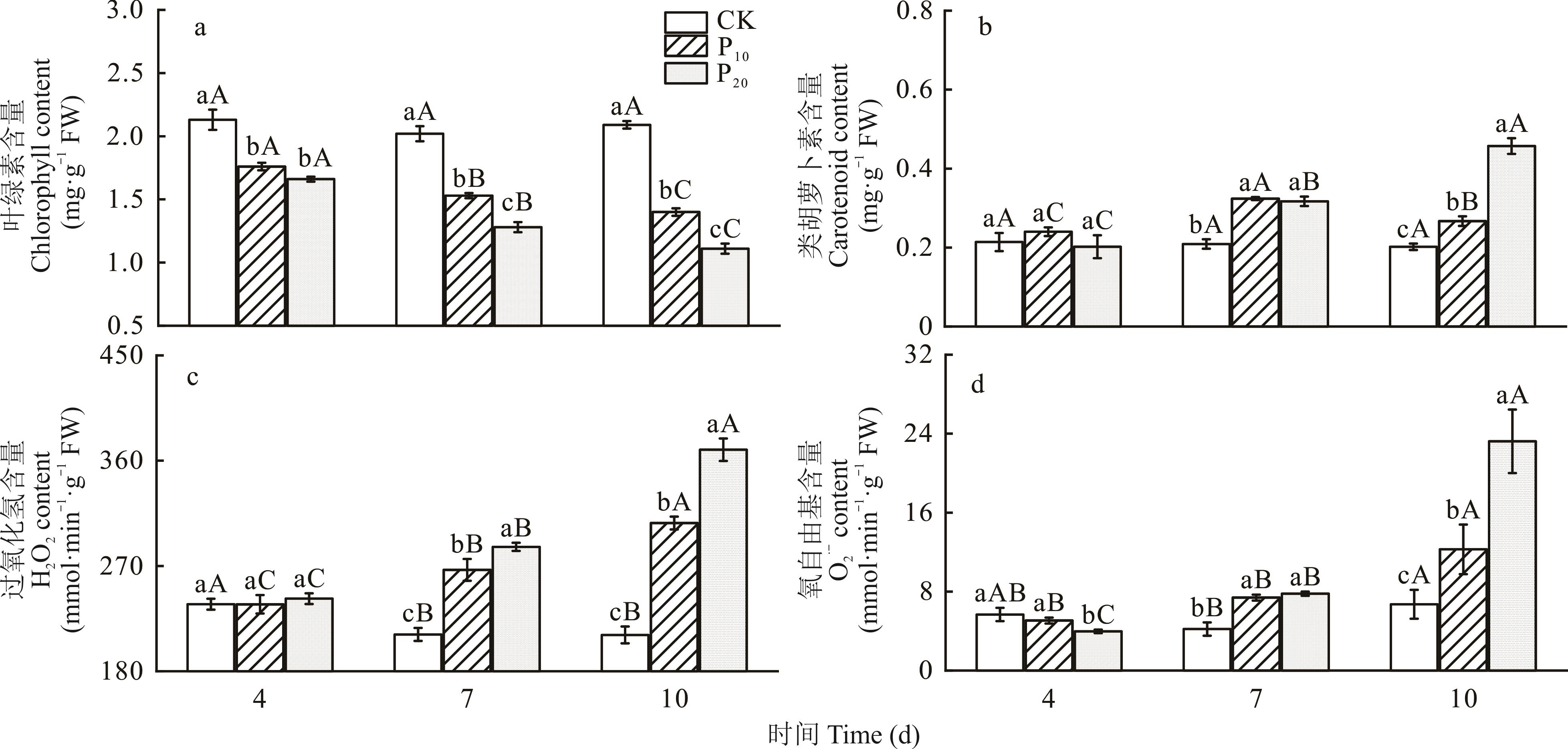
图1 不同水分胁迫下燕麦叶片生理指标变化不同小写字母表示同一干旱时间下不同干旱程度间差异显著(P<0.05),不同大写字母表示同一干旱程度下不同干旱时间差异显著(P<0.05)。下同。Different lowercase letters indicate significant differences between different drought degree under the same drought time(P<0.05), Capital letters indicate significant differences between different drought time under the same drought degree(P<0.05). The same below.
Fig.1 Effects of different drought stress on leaf physiological indicators of oat
| 因素Factors | 净光合速率Pn | 蒸腾速率Tr | 气孔导度Gs | 胞间CO2浓度Ci |
|---|---|---|---|---|
| 干旱时间Drought time (DT) | 510.775** | 104.285** | 131.488** | 102.176** |
| 干旱程度Drought degree (DD) | 1412.088** | 243.187** | 512.582** | 329.770** |
| 干旱时间×干旱程度DT×DD | 134.985** | 14.767** | 37.173** | 20.407** |
表2 干旱胁迫对燕麦气体交换参数影响的方差分析
Table 2 The variance analysis of effects on oat gas exchange parameters under drought stress
| 因素Factors | 净光合速率Pn | 蒸腾速率Tr | 气孔导度Gs | 胞间CO2浓度Ci |
|---|---|---|---|---|
| 干旱时间Drought time (DT) | 510.775** | 104.285** | 131.488** | 102.176** |
| 干旱程度Drought degree (DD) | 1412.088** | 243.187** | 512.582** | 329.770** |
| 干旱时间×干旱程度DT×DD | 134.985** | 14.767** | 37.173** | 20.407** |
指标 Index | 干旱时间 Drought time (d) | 干旱程度Drought degree | ||
|---|---|---|---|---|
| CK | P10 | P20 | ||
净光合速率Pn (μmol·m-2·s-1) | 4 | 15.33±0.03aA | 13.54±0.10aB | 8.82±0.26aC |
| 7 | 16.04±1.57aA | 9.33±0.22bB | 4.18±0.36bC | |
| 10 | 15.20±0.81aA | 1.94±0.09cB | 0.31±0.07cC | |
蒸腾速率Tr (mmol·m-2·s-1) | 4 | 4.61±0.47aA | 4.06±0.32aA | 1.90±0.09aB |
| 7 | 3.90±0.14aA | 2.36±0.20bB | 0.92±0.13bC | |
| 10 | 3.70±0.60aA | 0.64±0.07cB | 0.26±0.01cB | |
气孔导度Gs (mol·m-2·s-1) | 4 | 0.28±0.03aA | 0.25±0.03aA | 0.10±0.00aB |
| 7 | 0.26±0.01aA | 0.10±0.01bB | 0.04±0.01bC | |
| 10 | 0.26±0.01aA | 0.02±0.00cB | 0.01±0.00cC | |
胞间CO2浓度Ci (μmmol·mol-1) | 4 | 288.95±8.06aA | 286.62±6.23aA | 238.39±5.06aB |
| 7 | 289.63±12.56aA | 233.01±3.61bB | 184.94±7.75bC | |
| 10 | 273.15±2.21aA | 235.63±8.88bB | 155.25±8.91cC | |
表3 不同干旱胁迫下燕麦气体交换参数的变化
Table 3 Changes of gas exchange parameters of oat under different water stress
指标 Index | 干旱时间 Drought time (d) | 干旱程度Drought degree | ||
|---|---|---|---|---|
| CK | P10 | P20 | ||
净光合速率Pn (μmol·m-2·s-1) | 4 | 15.33±0.03aA | 13.54±0.10aB | 8.82±0.26aC |
| 7 | 16.04±1.57aA | 9.33±0.22bB | 4.18±0.36bC | |
| 10 | 15.20±0.81aA | 1.94±0.09cB | 0.31±0.07cC | |
蒸腾速率Tr (mmol·m-2·s-1) | 4 | 4.61±0.47aA | 4.06±0.32aA | 1.90±0.09aB |
| 7 | 3.90±0.14aA | 2.36±0.20bB | 0.92±0.13bC | |
| 10 | 3.70±0.60aA | 0.64±0.07cB | 0.26±0.01cB | |
气孔导度Gs (mol·m-2·s-1) | 4 | 0.28±0.03aA | 0.25±0.03aA | 0.10±0.00aB |
| 7 | 0.26±0.01aA | 0.10±0.01bB | 0.04±0.01bC | |
| 10 | 0.26±0.01aA | 0.02±0.00cB | 0.01±0.00cC | |
胞间CO2浓度Ci (μmmol·mol-1) | 4 | 288.95±8.06aA | 286.62±6.23aA | 238.39±5.06aB |
| 7 | 289.63±12.56aA | 233.01±3.61bB | 184.94±7.75bC | |
| 10 | 273.15±2.21aA | 235.63±8.88bB | 155.25±8.91cC | |
| 因素Factors | 初始荧光量Fo | 最大光化学效率Fv/Fm | 实际光化学效率ΦPSⅡ | 非光化学猝灭NPQ |
|---|---|---|---|---|
| 干旱时间Drought time (DT) | 175.315** | 12.517** | 62.283** | 21.924** |
| 干旱程度Drought degree (DD) | 0.272 | 27.036** | 121.852** | 33.601** |
| 干旱时间×干旱程度DT×DD | 40.407** | 7.484** | 13.504** | 7.481** |
表4 不同干旱胁迫对燕麦幼苗荧光特性影响的方差分析
Table 4 The variance analysis of effects on oat chlorophyll fluorescence characteristics under drought stress
| 因素Factors | 初始荧光量Fo | 最大光化学效率Fv/Fm | 实际光化学效率ΦPSⅡ | 非光化学猝灭NPQ |
|---|---|---|---|---|
| 干旱时间Drought time (DT) | 175.315** | 12.517** | 62.283** | 21.924** |
| 干旱程度Drought degree (DD) | 0.272 | 27.036** | 121.852** | 33.601** |
| 干旱时间×干旱程度DT×DD | 40.407** | 7.484** | 13.504** | 7.481** |
指标 Index | 干旱时间 Drought time (d) | 干旱程度Drought degree | ||
|---|---|---|---|---|
| CK | P10 | P20 | ||
初始荧光量 Fo | 4 | 156.40±11.70aB | 168.98±4.26aAB | 181.51±3.50aA |
| 7 | 154.76±1.37aB | 169.72±5.70aA | 171.98±2.00bA | |
| 10 | 150.22±5.00aA | 125.43±3.20bB | 105.13±4.21cC | |
最大光化学效率 Fv/Fm | 4 | 0.80±0.12aA | 0.81±0.11aA | 0.80±0.01aA |
| 7 | 0.82±0.10aA | 0.81±0.10aA | 0.74±0.02bB | |
| 10 | 0.80±0.00aA | 0.78±0.01bB | 0.69±0.05cC | |
实际光化学效率 ΦPSⅡ | 4 | 0.46±0.01aA | 0.42±0.03aA | 0.33±0.06aB |
| 7 | 0.43±0.01aA | 0.35±0.02bB | 0.24±0.01bC | |
| 10 | 0.44±0.05aA | 0.17±0.01cB | 0.14±0.02cB | |
非光化学猝灭 NPQ | 4 | 0.76±0.03aB | 0.71±0.10bB | 1.06±0.03cA |
| 7 | 0.68±0.18aB | 1.14±0.45bAB | 1.43±0.19bA | |
| 10 | 0.72±0.02aB | 1.78±0.09aA | 1.69±0.03aA | |
表5 不同水分胁迫下燕麦荧光参数的变化
Table 5 Changes of fluorescence parameters of oat under different water stress
指标 Index | 干旱时间 Drought time (d) | 干旱程度Drought degree | ||
|---|---|---|---|---|
| CK | P10 | P20 | ||
初始荧光量 Fo | 4 | 156.40±11.70aB | 168.98±4.26aAB | 181.51±3.50aA |
| 7 | 154.76±1.37aB | 169.72±5.70aA | 171.98±2.00bA | |
| 10 | 150.22±5.00aA | 125.43±3.20bB | 105.13±4.21cC | |
最大光化学效率 Fv/Fm | 4 | 0.80±0.12aA | 0.81±0.11aA | 0.80±0.01aA |
| 7 | 0.82±0.10aA | 0.81±0.10aA | 0.74±0.02bB | |
| 10 | 0.80±0.00aA | 0.78±0.01bB | 0.69±0.05cC | |
实际光化学效率 ΦPSⅡ | 4 | 0.46±0.01aA | 0.42±0.03aA | 0.33±0.06aB |
| 7 | 0.43±0.01aA | 0.35±0.02bB | 0.24±0.01bC | |
| 10 | 0.44±0.05aA | 0.17±0.01cB | 0.14±0.02cB | |
非光化学猝灭 NPQ | 4 | 0.76±0.03aB | 0.71±0.10bB | 1.06±0.03cA |
| 7 | 0.68±0.18aB | 1.14±0.45bAB | 1.43±0.19bA | |
| 10 | 0.72±0.02aB | 1.78±0.09aA | 1.69±0.03aA | |
因素 Factors | 超氧化物 歧化酶SOD | 过氧化氢酶 CAT | 抗坏血酸过 氧化物酶APX | 谷胱甘肽过氧 化物酶GPX | 脱氢抗坏血酸 还原酶DHAR | 谷胱甘肽 还原酶GR |
|---|---|---|---|---|---|---|
| 干旱时间Drought time (DT) | 670.452** | 2.413 | 0.649 | 23.032** | 21.719** | 5.998 |
| 干旱程度Drought degree (DD) | 601.082** | 89.800** | 30.032** | 17.910** | 29.933** | 44.709 |
| 干旱时间×干旱程度DT×DD | 244.314** | 49.034** | 32.713** | 72.259** | 8.202** | 68.187** |
表6 不同干旱胁迫对燕麦抗氧化酶活性的方差分析
Table 6 The variance analysis of effects on activity of enzymatic antioxidants under different drought stress
因素 Factors | 超氧化物 歧化酶SOD | 过氧化氢酶 CAT | 抗坏血酸过 氧化物酶APX | 谷胱甘肽过氧 化物酶GPX | 脱氢抗坏血酸 还原酶DHAR | 谷胱甘肽 还原酶GR |
|---|---|---|---|---|---|---|
| 干旱时间Drought time (DT) | 670.452** | 2.413 | 0.649 | 23.032** | 21.719** | 5.998 |
| 干旱程度Drought degree (DD) | 601.082** | 89.800** | 30.032** | 17.910** | 29.933** | 44.709 |
| 干旱时间×干旱程度DT×DD | 244.314** | 49.034** | 32.713** | 72.259** | 8.202** | 68.187** |
因素 Factors | 抗坏血酸 ASA | 谷胱甘肽 GSH | 脱氢抗坏血酸DHA | 氧化型谷胱甘肽GSSG | 抗坏血酸清除能力ASA/DHA | 谷胱甘肽清除能力GSH/GSSG |
|---|---|---|---|---|---|---|
| 干旱时间Drought time (DT) | 196.468** | 43.573** | 4.183* | 65.876** | 31.198** | 12.772** |
| 干旱程度Drought degree (DD) | 229.823** | 177.413** | 51.824** | 84.209** | 18.961** | 4.688* |
| 干旱时间×干旱程度DT×DD | 75.408** | 11.303** | 2.471 | 11.602** | 19.176** | 2.859 |
表7 不同干旱胁迫对燕麦非酶抗氧化剂影响的方差分析
Table 7 The variance analysis of effects on activity of non-enzymatic antioxidants under different drought stress
因素 Factors | 抗坏血酸 ASA | 谷胱甘肽 GSH | 脱氢抗坏血酸DHA | 氧化型谷胱甘肽GSSG | 抗坏血酸清除能力ASA/DHA | 谷胱甘肽清除能力GSH/GSSG |
|---|---|---|---|---|---|---|
| 干旱时间Drought time (DT) | 196.468** | 43.573** | 4.183* | 65.876** | 31.198** | 12.772** |
| 干旱程度Drought degree (DD) | 229.823** | 177.413** | 51.824** | 84.209** | 18.961** | 4.688* |
| 干旱时间×干旱程度DT×DD | 75.408** | 11.303** | 2.471 | 11.602** | 19.176** | 2.859 |
| 1 | Chaves M M, Pereira J S, Maroco J, et al. How plants cope with water stress in the field. Photosynthesis and Growth. Annals of Botany, 2002, 89(7): 907-916. |
| 2 | Zhang L, Xin Z, Yu X, et al. Osmotic stress induced cell death in heat is alleviated by tauroursodeoxycholic acid and involves edoplasmic reticulum stress-Related gene expression. Frontiers in Plant Science, 2017, 1(8): 667. |
| 3 | Lourens P, Frans B. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology, 2006, 87(7): 1733-1743. |
| 4 | Freschet G T, Cornelissen J H C, Logtestijn R S P V, et al. Evidence of the ‘plant economics spectrum’ in a subarctic flora. Journal of Ecology, 2010, 98(2): 362-373. |
| 5 | Habap D L, Molina E, Matal D L, et al. High temperature promotes early senescence in primary leaves of sunflower (Helianthus annuus L.) plants. Canadian Journal of Plant Science, 2014, 94(4): 659-669. |
| 6 | Xing X, Zhou Q, Xing H, et al. Early abscisic acid accumulation regulates ascorbate and glutathione metabolism in soybean leaves under progressive water stress. Journal of Plant Growth Regulation, 2016, 35(3): 865-876. |
| 7 | Lin D D, Zhao G Q, Ju Z L, et al. Comprehensive evaluation of drought resistance of 15 oat varieties at the seedling stage. Acta Prataculturae Sinica, 2021, 30(11): 108-121. |
| 蔺豆豆, 赵桂琴, 琚泽亮, 等. 15份燕麦材料苗期抗旱性综合评价. 草业学报, 2021, 30(11): 108-121. | |
| 8 | Sun A, Deng H F, Li K Y, et al. Effects of PEG stress on enzyme activity of oat seedling. Journal of Domestic Animal Ecology, 2012, 33(1): 50-52. |
| 孙鏖, 邓荟芬, 李科云, 等. PEG胁迫对燕麦苗期保护酶的影响. 家畜生态学报, 2012, 33(1): 50-52. | |
| 9 | Ehlers W. Transpirations efficiency of oat. Agronomy Journal, 1989, 81(5): 810-817. |
| 10 | Islam M R, Ren C Z, Zeng Z H, et al. Fertilizer use efficiency of drought-stressed oat (Avena sativa L.) following soil amendment with a water-saving superabsorbent polymer. Acta Agriculturae Scandinavica, Section B-Soil & Plant Science, 2011, 61(8): 721-729. |
| 11 | Zhao G Q, Shi S L.The current situation of oat research and production, problems and strategy in Tibetan Plateau. Pratacultural Science, 2004, 21(11): 17-21. |
| 赵桂琴, 师尚礼. 青藏高原饲用燕麦研究与生产现状,存在问题与对策. 草业科学, 2004, 21(11): 17-21. | |
| 12 | Cui X X, Hou F J, Chang S H, et al. Comparison of yield and nutritional quality of two oat (Avena sativa) varieties grown in the alpine pastoral region of China. Pratacultural Science, 2018, 35(6): 1489-1495. |
| 崔雄雄, 侯扶江, 常生华, 等. 高寒牧区两个燕麦品种的产量与品质比较. 草业科学, 2018, 35(6): 1489-1495. | |
| 13 | Liu W Y, Zhou F, Yang R Q, et al. A study of Avena nuda L. seeding under drought stress. Journal of Shanxi Datong University (Natural Science Edition), 2013, 29(4): 53-55, 75. |
| 刘文英, 周凤, 杨瑞卿, 等. 干旱胁迫对裸燕麦幼苗生长的影响. 山西大同大学学报(自然科学版), 2013, 29(4): 53-55, 75. | |
| 14 | Liu J X, Wang J C, Wang R J, et al. Effects of nitric oxide on growth and physiological characteristics of oat seedlings under drought stress. Chinese Journal of Grassland, 2015, 37(2): 41-45. |
| 刘建新, 王金成, 王瑞娟, 等. 干旱胁迫下一氧化氮对燕麦幼苗生长和生理特性的影响. 中国草地学报, 2015, 37(2): 41-45. | |
| 15 | Qi H, Xu J, Meng X H, et al. Study on photosynthetic characteristics of oat in seedling stage under water stress.Agricultural Science and Technology Newsletter, 2009(5): 31-34. |
| 齐华, 许晶, 孟显华, 等. 水分胁迫对燕麦苗期光合特性的影响. 农业科技通讯, 2009(5): 31-34. | |
| 16 | Wang D. Exogenously applied trehalose protects the structure and function of photosystem Ⅱ (PSⅡ) under heat stress. Shanghai: East China Normal University, 2016. |
| 王迪. 外源海藻糖在高温胁迫下保护光系统Ⅱ的结构和功能. 上海: 华东师范大学, 2016. | |
| 17 | Schneider K, Schlegel H G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochemical Journal, 1981, 193(1): 99-107. |
| 18 | Liu Z J,Guo Y K, Bai J G. Exogenous hydrogen peroxide changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber ecotypes under osmotic stress. Journal of Plant Growth Regulation, 2010, 29(2): 171-183. |
| 19 | Asish K P A, Anath B D A B, Prasanna M B. Defense potentials to NaCl in a mangrove, Bruguiera parviflora: Differential changes of isoforms of some antioxidative enzymes. Journal of Plant Physiology, 2004, 161(5): 531-542. |
| 20 | Zhang J, Kirkham M B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist, 1996, 132(3): 361-373. |
| 21 | Yoshiyuki N, Kozi A. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiology, 1980, 22(5): 867-880. |
| 22 | Elia A C, Galarini R, Taticchi M I, et al. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicology & Environmental Safety, 2003, 55(2): 162-167. |
| 23 | Foyer C H, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta, 1976, 133(1): 21-25. |
| 24 | Niu K J. The regulatory mechanism of exogenous 5-aminolevulinic acid on the photosynthesis of Poa pratensis under drought stress. Lanzhou: Gansu Agricultural University, 2018. |
| 牛奎举. 外源5-氨基乙酰丙酸对干旱胁迫下草地早熟禾光合作用的调控机制. 兰州: 甘肃农业大学, 2018. | |
| 25 | Yi F Y, Peng K L. Study on the growth characteristics and culture utilization of Eohinochloa crusgalli. Pratacultural Science, 1993, 10(5): 62-64. |
| 易凤银, 彭科林. 饲用稗草生育特性及栽培利用的研究. 草业科学, 1993, 10(5): 62-64. | |
| 26 | Pack A I. Current opinion in biotechnology. Elsevier Science, 1998, 3(6): 3-9. |
| 27 | Zhang H. Physiological and quantitative proteomics study of chloroplast response to Na2CO3 stress in Puccinellia tenuiflora. Harbin: Northeast Forestry University, 2012. |
| 张恒. 星星草(Puccinellia tenuiflora)叶绿体Na2CO3胁迫应答的生理学与定量蛋白质组学研究. 哈尔滨: 东北林业大学, 2012. | |
| 28 | Wu X L. Studies on physiological characteristics of different alfalfa varieties. Xianyang: Northwest A & F University, 2008. |
| 吴晓丽. 不同紫花苜蓿品种的抗旱生理特性比较研究. 咸阳: 西北农林科技大学, 2008. | |
| 29 | Xu Y P, Hu C M, Zhang W H, et al. Effect of simulated drought stress on photosynthesis related indexes at seedling stage of wild soybeans. Soybean Science, 2013, 32(3): 341-344. |
| 徐艳平, 胡翠美, 张文会, 等. 干旱胁迫对野生大豆幼苗光合作用相关指标的影响. 大豆科学, 2013, 32(3): 341-344. | |
| 30 | Schreiber U, Bilger W, Neubauer C. Chlorophyll fluorescence as a nonintrusive indicator for rapid sessment of in vivo photosynthesis. Berlin, Heidelberg: Springer-Verlag, 1995. |
| 31 | Genty B, Briantai J M, Baker N R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-General Subjects, 1989, 990(1): 87-92. |
| 32 | Chen M Y. Responses and physiological mechanism of seedling growth of two provenances of quercus variabilis to drought stress. Beijing: Chinese Academy of Forestry, 2019. |
| 陈梦园. 两个种源栓皮栎幼苗生长对干旱胁迫的响应及生理机制. 北京: 中国林业科学研究院, 2019. | |
| 33 | Kate M, Johnson G N. Chlorophyll fluorescence-A practical guide. Journal of Experimental Botany, 2000, 51(345): 659-668. |
| 34 | Reinbothe S, Reinbothe C. The regulation of enzymes involved in chlorophyll biosynthesis. European Journal of Biochemistry, 1996, 237(2): 323-343. |
| 35 | Gao C T. Appraisal and appliance of barley germplasm resources. Hohhot: Inner Mongolia Agricultural University, 2005. |
| 高彩婷. 大麦种质资源评价与利用. 呼和浩特: 内蒙古农业大学, 2005. | |
| 36 | Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 2004, 55(1): 373-399. |
| 37 | Bai J, Tai K, Wu H, et al. Relative contribution of photorespiration and antioxidative mechanisms in Caragana korshinskii under drought conditions across the Loess Plateau. Functional Plant Biology, 2017, 44(11): 10.1071/FP17060. |
| 38 | Han Y S. Advances of the function of beta-carotene and carotenoid. Journal of China Agricultural University, 1999, 4(1): 5-9. |
| 韩雅珊. 类胡萝卜素的功能研究进展. 中国农业大学学报, 1999, 4(1): 5-9. | |
| 39 | Dong S K, Ma Y L, Li S, et al. Effect of drought stress and re-watering on ascorbate-glutathionecycle of soybean. Journal of Northeast Agricultural University, 2018, 49(1): 10-18. |
| 董守坤, 马玉玲, 李爽, 等. 干旱胁迫及复水对大豆抗坏血酸-谷胱甘肽循环的影响. 东北农业大学学报, 2018, 49(1): 10-18. |
| [1] | 田吉鹏, 刘蓓一, 顾洪如, 丁成龙, 程云辉, 玉柱. 乳酸菌及丙酸钙对全株玉米和燕麦青贮饲料发酵品质和霉菌毒素含量的影响[J]. 草业学报, 2022, 31(8): 157-166. |
| [2] | 曾令霜, 李培英, 孙宗玖, 孙晓梵. 两类新疆狗牙根抗旱基因型抗氧化酶保护系统及其基因表达差异分析[J]. 草业学报, 2022, 31(7): 122-132. |
| [3] | 金祎婷, 刘文辉, 刘凯强, 梁国玲, 贾志锋. 全生育期干旱胁迫对‘青燕1号’燕麦叶绿素荧光参数的影响[J]. 草业学报, 2022, 31(6): 112-126. |
| [4] | 苏世平, 李毅, 刘小娥, 种培芳, 单立山, 后有丽. 外源脯氨酸对缓解红砂干旱胁迫的机理研究[J]. 草业学报, 2022, 31(6): 127-138. |
| [5] | 孙晓梵, 张一龙, 李培英, 孙宗玖. 不同施氮量对干旱下狗牙根抗氧化酶活性及渗透调节物质含量的影响[J]. 草业学报, 2022, 31(6): 69-78. |
| [6] | 蔺豆豆, 琚泽亮, 柴继宽, 赵桂琴. 青藏高原燕麦附着耐低温乳酸菌的筛选与鉴定[J]. 草业学报, 2022, 31(5): 103-114. |
| [7] | 李满有, 杨彦军, 王斌, 沈笑天, 曹立娟, 李小云, 倪旺, 兰剑. 宁夏干旱区滴灌条件下燕麦与光叶紫花苕不同混播模式的生产性能、品质及综合评价研究[J]. 草业学报, 2022, 31(4): 62-71. |
| [8] | 吴海艳, 曲尼, 曲珍, 同桑措姆, 达娃卓嘎, 德央, 尼玛卓嘎, 刘昭明, 马玉寿. 6个燕麦品种在昂仁县的生产性能及饲草品质比较[J]. 草业学报, 2022, 31(4): 72-80. |
| [9] | 沈吉成, 王蕾, 赵彩霞, 叶发慧, 吕士凯, 刘德梅, 刘瑞娟, 张怀刚, 陈文杰. 77份裸燕麦品种籽粒相关性状分析[J]. 草业学报, 2022, 31(3): 156-167. |
| [10] | 王志恒, 魏玉清, 赵延蓉, 王悦娟. 基于转录组学比较研究甜高粱幼苗响应干旱和盐胁迫的生理特征[J]. 草业学报, 2022, 31(3): 71-84. |
| [11] | 高鹏飞, 张静, 范卫芳, 高冰, 郝宏娟, 吴建慧. 干旱胁迫对光叉委陵菜根系特征、结构和生理特性的影响[J]. 草业学报, 2022, 31(2): 203-212. |
| [12] | 张鹏, 任茜, 孟思宇, 魏小星, 鲍根生. 内生真菌对盐胁迫下紫花针茅种子萌发和幼苗生长的研究[J]. 草业学报, 2022, 31(10): 110-121. |
| [13] | 魏娜, 李艳鹏, 马艺桐, 刘文献. 全基因组水平紫花苜蓿TCP基因家族的鉴定及其在干旱胁迫下表达模式分析[J]. 草业学报, 2022, 31(1): 118-130. |
| [14] | 赵颖, 辛夏青, 魏小红. 一氧化氮对干旱胁迫下紫花苜蓿氮代谢的影响[J]. 草业学报, 2021, 30(9): 86-96. |
| [15] | 汪雪, 刘晓静, 赵雅姣, 王静. 根系分隔方式下紫花苜蓿/燕麦间作氮素利用及种间互馈特征研究[J]. 草业学报, 2021, 30(8): 73-85. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||