

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2024, Vol. 33 ›› Issue (1): 75-88.DOI: 10.11686/cyxb2023085
谭炯锐1( ), 查同刚2(
), 查同刚2( ), 张泽宇2, 张晓霞3, 滕红梅1, 王玲丽1, 赵莉丽4, 王奥4, 王馨珧1
), 张泽宇2, 张晓霞3, 滕红梅1, 王玲丽1, 赵莉丽4, 王奥4, 王馨珧1
收稿日期:2023-03-21
修回日期:2023-05-31
出版日期:2024-01-20
发布日期:2023-11-23
通讯作者:
查同刚
作者简介:E-mail: zhtg73@bjfu.edu.cn基金资助:
Jiong-rui TAN1( ), Tong-gang ZHA2(
), Tong-gang ZHA2( ), Ze-yu ZHANG2, Xiao-xia ZHANG3, Hong-mei TENG1, Ling-li WANG1, Li-li ZHAO4, Ao WANG4, Xin-yao WANG1
), Ze-yu ZHANG2, Xiao-xia ZHANG3, Hong-mei TENG1, Ling-li WANG1, Li-li ZHAO4, Ao WANG4, Xin-yao WANG1
Received:2023-03-21
Revised:2023-05-31
Online:2024-01-20
Published:2023-11-23
Contact:
Tong-gang ZHA
摘要:
为探究猪毛菜在表型、生理及分子层面对干旱胁迫的响应机制,采用控水法进行梯度干旱胁迫,对叶片进行解剖结构和生理指标测定及转录组测序。结果表明:干旱胁迫导致栅栏组织从致密变稀疏,晶簇先消失后增多;贮水组织厚度先增大后降低(P<0.05),主维管束面积和表皮厚度在28 d时显著增大,分别为(3099.72±151.88) μm2和(23.73±0.68) μm(P<0.05),叶片厚度增大而叶面积减小(P<0.05);叶绿素含量逐渐降低(P<0.05),超氧化物歧化酶(SOD)和过氧化物酶(POD)活性、脯氨酸和丙二醛(MDA)含量在28 d显著增大(P<0.05),H2O2含量在14 d显著增大(P<0.05),可溶性蛋白含量先增后降再增(P<0.05)。与0 d比较,干旱胁迫7、14和28 d的差异表达基因(DEGs)分别有103、1560和270个;基因本体(GO)富集分析表明各组合DEGs显著富集在蛋白质磷酸酶Ⅰ型复合体、膜的整体组件、氧化还原过程、氧化还原酶活性等条目中;京都基因与基因组百科全书(KEGG)富集分析表明各组合DEGs同时显著富集在氧化磷酸化代谢、核糖体和光传导等通路中。非生物胁迫通路DEGs分析表明,热激蛋白基因、GPX2、POD、SOD、CPK19、CIPK9、MAP激酶基因、蛋白质降解通路基因、UXS1和F3H等参与对干旱胁迫的响应调节。研究结果可为进一步研究猪毛菜抗旱机制提供参考。
谭炯锐, 查同刚, 张泽宇, 张晓霞, 滕红梅, 王玲丽, 赵莉丽, 王奥, 王馨珧. 猪毛菜响应干旱胁迫的叶片结构、生理及转录组分析[J]. 草业学报, 2024, 33(1): 75-88.
Jiong-rui TAN, Tong-gang ZHA, Ze-yu ZHANG, Xiao-xia ZHANG, Hong-mei TENG, Ling-li WANG, Li-li ZHAO, Ao WANG, Xin-yao WANG. Leaf structure, physiology and transcriptome analysis of Salsola collina in response to drought stress[J]. Acta Prataculturae Sinica, 2024, 33(1): 75-88.
| 基因编号Gene ID | 正向引物Forward primers | |
|---|---|---|
| TR16542_c0_g1 | TGCTAACGGTTGGAGATGCT | TGCAGCAATTTTGGGGTTGC |
| TR17024_c0_g1 | CGTTGTCGTCGTTGCTCTTC | CCTATCCGATCCATGGCAGG |
| TR17405_c0_g1 | TTGGGCTAGTGATGGAGGGA | GCAATTGGGATCCGAACACG |
| TR2171_c0_g1 | AAGAAACCACGAAACGCACG | GTGTGTCGGTTCAAGTTGCC |
| TR5292_c0_g1 | GTGGAGCTTGCCTCCAAAGA | CATGGGTCCCACATCGACTC |
| TR7123_c0_g1 | ACTATCGGGGGTAGCCAACT | CGAGCTTATTGGCCTCGGAT |
| EF1A | TGGTCGTTTTGCTGTGAGGG | GCAGCCTTGGTCACCTTTG |
表1 差异表达基因引物序列
Table 1 Primers of differentially expressed genes
| 基因编号Gene ID | 正向引物Forward primers | |
|---|---|---|
| TR16542_c0_g1 | TGCTAACGGTTGGAGATGCT | TGCAGCAATTTTGGGGTTGC |
| TR17024_c0_g1 | CGTTGTCGTCGTTGCTCTTC | CCTATCCGATCCATGGCAGG |
| TR17405_c0_g1 | TTGGGCTAGTGATGGAGGGA | GCAATTGGGATCCGAACACG |
| TR2171_c0_g1 | AAGAAACCACGAAACGCACG | GTGTGTCGGTTCAAGTTGCC |
| TR5292_c0_g1 | GTGGAGCTTGCCTCCAAAGA | CATGGGTCCCACATCGACTC |
| TR7123_c0_g1 | ACTATCGGGGGTAGCCAACT | CGAGCTTATTGGCCTCGGAT |
| EF1A | TGGTCGTTTTGCTGTGAGGG | GCAGCCTTGGTCACCTTTG |

图1 干旱胁迫下猪毛菜叶片解剖结构a~d代表停止供水0、7、14和28 d。a-d represent 0, 7, 14 and 28 days without water supply. BS: 维管束鞘Vascular bundle sheath; PM: 栅栏组织Palisade tissue; VB: 维管束Vascular bundle; WS: 贮水组织Water aqueous tissue.
Fig.1 Anatomical structures of the leaves of S. collina under drought stress
胁迫天数 Stress days (d) | 栅栏组织厚度 Thickness of palisade tissue (μm) | 贮水组织厚度 Thickness of aqueous tissue (μm) | 主维管束面积 Area of main vascular bundle (μm2) | 表皮厚度 Epidermal thickness (μm) | 叶片厚度 Leaf thickness (μm) | 叶面积 Leaf area (mm2) |
|---|---|---|---|---|---|---|
| 0 | 43.15±3.49a | 149.66±19.62b | 2065.41±80.08b | 13.71±1.26b | 506.29±3.50b | 42.22±2.65a |
| 7 | 52.30±2.28a | 210.09±15.68a | 2374.54±218.09b | 15.03±0.97b | 573.27±17.04a | 30.46±1.89b |
| 14 | 43.23±3.31a | 166.67±8.20ab | 2776.56±155.15ab | 16.08±2.64b | 554.08±24.05a | 37.41±2.18ab |
| 28 | 38.89±6.13b | 140.55±18.48b | 3099.72±151.88a | 23.73±0.68a | 531.21±21.83a | 36.40±2.94ab |
表2 猪毛菜叶片解剖结构各参数随干旱胁迫的变化
Table 2 Changes of anatomical parameters of leaves of S. collina with drought stress
胁迫天数 Stress days (d) | 栅栏组织厚度 Thickness of palisade tissue (μm) | 贮水组织厚度 Thickness of aqueous tissue (μm) | 主维管束面积 Area of main vascular bundle (μm2) | 表皮厚度 Epidermal thickness (μm) | 叶片厚度 Leaf thickness (μm) | 叶面积 Leaf area (mm2) |
|---|---|---|---|---|---|---|
| 0 | 43.15±3.49a | 149.66±19.62b | 2065.41±80.08b | 13.71±1.26b | 506.29±3.50b | 42.22±2.65a |
| 7 | 52.30±2.28a | 210.09±15.68a | 2374.54±218.09b | 15.03±0.97b | 573.27±17.04a | 30.46±1.89b |
| 14 | 43.23±3.31a | 166.67±8.20ab | 2776.56±155.15ab | 16.08±2.64b | 554.08±24.05a | 37.41±2.18ab |
| 28 | 38.89±6.13b | 140.55±18.48b | 3099.72±151.88a | 23.73±0.68a | 531.21±21.83a | 36.40±2.94ab |
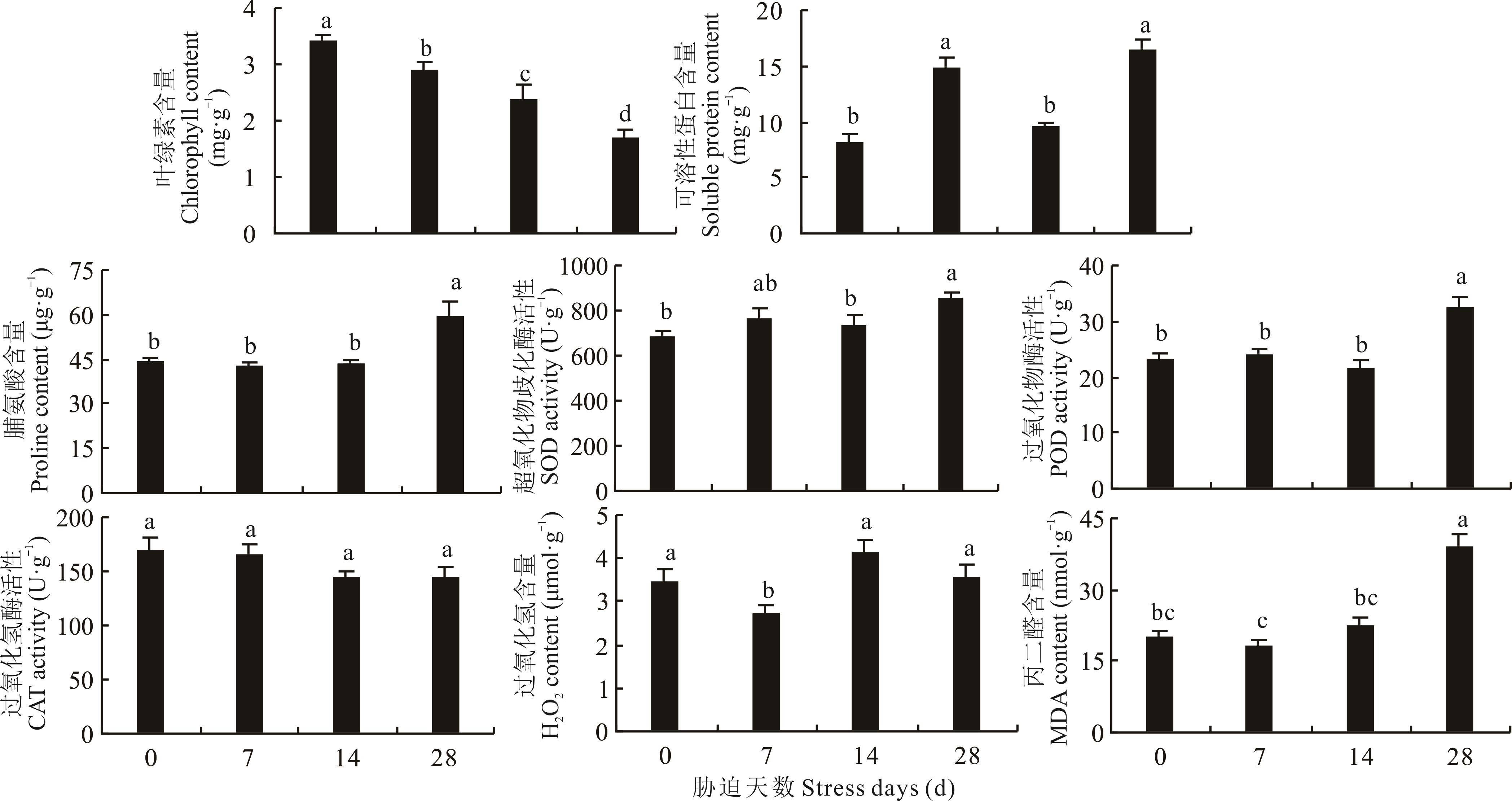
图2 猪毛菜叶片生理指标随干旱胁迫的变化不同小写字母表示不同胁迫时间处理间差异显著(P<0.05)。Different lowercase letters indicate significant differences among the different drought stress time treatments at 0.05 level.
Fig.2 Changes of physiological indexes of leaves of S. collina with drought stress
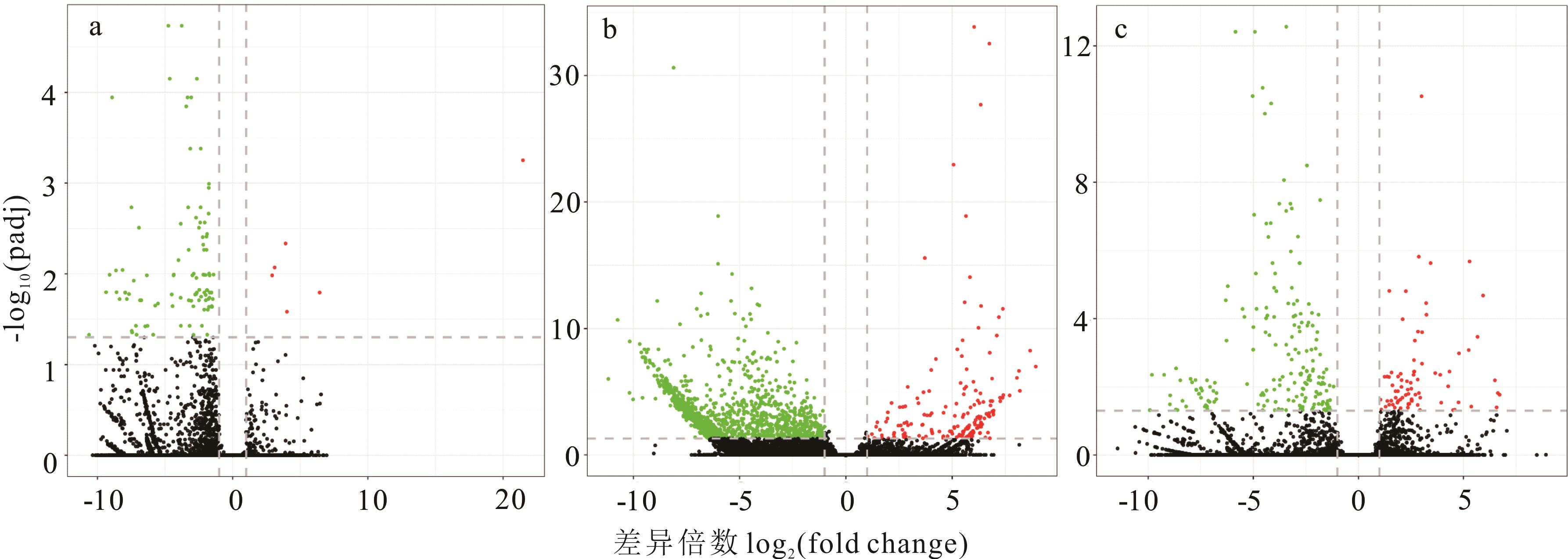
图3 不同程度干旱胁迫差异表达基因火山图红点代表上调表达,绿点代表下调表达,黑点代表表达无显著差异。Red dots represent up-regulated expression, green dots represent down-regulated expression, and black dots represent no significant difference in expression. a: 7 d Vs 0 d; b:14 d Vs 0 d; c: 28 d Vs 0 d. 下同The same below.
Fig.3 Volcanic diagram of differentially expressed genes (DEGs) under different degrees of drought stress
项目 Item | GO编号 GO ID | 条目 Term | 种类 Category | 基因数目 Gene number | P值 P value |
|---|---|---|---|---|---|
| 7 d Vs 0 d | 0035966 | BP | 4 | 0.00 | |
| 0030968 | BP | 3 | 0.00 | ||
| 0034620 | BP | 3 | 0.00 | ||
| 0035967 | BP | 3 | 0.00 | ||
| 0006986 | BP | 3 | 0.00 | ||
| 1 | 0.00 | ||||
| 1 | 0.00 | ||||
表3 差异表达基因GO富集前5名条目
Table 3 Top 5 terms of GO enrichment analysis of the differentially expressed genes
项目 Item | GO编号 GO ID | 条目 Term | 种类 Category | 基因数目 Gene number | P值 P value |
|---|---|---|---|---|---|
| 7 d Vs 0 d | 0035966 | BP | 4 | 0.00 | |
| 0030968 | BP | 3 | 0.00 | ||
| 0034620 | BP | 3 | 0.00 | ||
| 0035967 | BP | 3 | 0.00 | ||
| 0006986 | BP | 3 | 0.00 | ||
| 1 | 0.00 | ||||
| 1 | 0.00 | ||||

图5 差异表达基因KEGG富集分析点的大小代表基因数目,点的颜色代表P值。The size of dots represents the number of genes and the color of dots represents P values.
Fig.5 KEGG enrichment analysis of differentially expressed genes

图7 胁迫相关通路基因表达随干旱胁迫的变化a: 7 d Vs 0 d; b: 14 d Vs 7 d; c: 28 d Vs 14 d. 方块代表差异表达基因,红色为上调,蓝色为下调。The squares represent differentially expressed genes, red represents up-regulated expression, blue represents down-regulated expression.
Fig.7 Gene expression of stress-related pathways changes with drought stress
| 1 | Diatta A A, Fike J H, Battaglia M L, et al. Effects of biochar on soil fertility and crop productivity in arid regions: a review. Arabian Journal of Geosciences, 2020, 13(14): 595. |
| 2 | O’connell E. Towards adaptation of water resource systems to climatic and socio-economic change. Water Resources Management, 2017, 31(10): 2965-2984. |
| 3 | Liu J S. Plant physiology. Beijing: Higher Education Press, 2006: 220-222. |
| 刘继澍. 植物生理学. 北京: 高等教育出版社, 2006: 220-222. | |
| 4 | Zhang H, Zhu J, Gong Z, et al. Abiotic stress responses in plants. Nature Reviews Genetics, 2022, 23(2): 104-119. |
| 5 | Hirt H. Connecting oxidative stress, auxin, and cell cycle regulation through a plant mitogen-activated protein kinase pathway. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(6): 2405-2407. |
| 6 | Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. Journal of Experimental Botany, 2014, 65(5): 1229-1240. |
| 7 | Xu Z, Jiang Y, Zhou G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Frontiers in Plant Science, 2015, 6: 701. |
| 8 | Mansoor S, Ali W O, Lone J K, et al. Reactive oxygen species in plants: from source to sink. Antioxidants, 2022, 11(2): 225. |
| 9 | Zhao Y, Wei X, Ji X, et al. Endogenous NO-mediated transcripts involved in photosynthesis and carbohydrate metabolism in alfalfa (Medicago sativa L.) seedlings under drought stress. Plant Physiology and Biochemistry, 2019, 141: 456-465. |
| 10 | Li S, Fan C, Li Y, et al. Effects of drought and salt-stresses on gene expression in Caragana korshinskii seedlings revealed by RNA-seq. BMC Genomics, 2016, 17(1): 200. |
| 11 | Yang F, Lv G. Combined analysis of transcriptome and metabolome reveals the molecular mechanism and candidate genes of Haloxylon drought tolerance. Frontiers in Plant Science, 2022, 13: 1020367. |
| 12 | Yan D H. Genome-wide transcriptional response and expressed NF-YB genes of Populus euphratica to drought stress. Beijing: Beijing Forestry University, 2013. |
| 严东辉. 胡杨干旱响应转录组及NF-YB基因表达谱. 北京: 北京林业大学, 2013. | |
| 13 | Duan N. Growth and physiological and transcriptomic studies in responses to nitrogen addition and drought stress in Nitraria tangutorum. Hohhot: Inner Mongolia Agricultural University, 2019. |
| 段娜. 白刺对氮添加和干旱胁迫的生长生理响应及转录组学研究. 呼和浩特: 内蒙古农业大学, 2019. | |
| 14 | Jin M, Su Y H. Identification and analysis on transcription factor genes from Ammopiptanthus mongolicus in responding to abiotic stress. Journal of Plant Resources and Environment, 2018, 27(1): 1-10. |
| 金曼, 苏彦华. 沙冬青响应非生物胁迫的转录因子基因鉴定与分析. 植物资源与环境学报, 2018, 27(1): 1-10. | |
| 15 | Bai W J. Effects of vegetation restoration on soil quality and plant physio-ecological adaptability in water-wind erosion region. Yangling: Chinese Academy of Sciences, 2010. |
| 白文娟. 水蚀风蚀交错带植被恢复对土壤质量的影响与植物生理生态适应性. 杨凌: 中国科学院, 2010. | |
| 16 | Peng L, Zhang L, Zhou X L, et al. Effects of water stress on life history strategy of Salsola nitraria in Zhundong, Xinjiang. Acta Prataculturae Sinica, 2021, 30(5): 65-74. |
| 彭磊, 张力, 周小龙, 等. 水分胁迫对新疆准东地区钠猪毛菜的生活史对策的影响. 草业学报, 2021, 30(5): 65-74. | |
| 17 | Wen Z B, Feng Y. Biodiversity and geographical distribution of the genus Salsola L. in Xinjiang. Arid Zone Research, 2020, 37(1): 185-192. |
| 闻志彬, 冯缨. 新疆猪毛菜属植物多样性及其地理分布特征. 干旱区研究, 2020, 37(1): 185-192. | |
| 18 | Chen P, Jiang L, Yang W, et al. Seed germination response and tolerance to different abiotic stresses of four Salsola species growing in an arid environment. Frontiers in Plant Science, 2022, 13: 892667. |
| 19 | Elnaggar R A, El-Keblawy A, Mosa K, et al. Adaptive drought tolerance during germination of Salsola drummondii seeds from saline and non-saline habitats of the arid Arabian Deserts. Botany, 2018, 97(2): 123-133. |
| 20 | Zhang Y H, Wang Y L, Xia C L, et al. Cloning and analysis of NADP-ME gene family and promoters in Salsola laricifolia. Acta Botanica Boreali-Occidentalia Sinica, 2022, 42(5): 760-769. |
| 张玉慧, 王玉兰, 夏春兰, 等. 松叶猪毛菜NADP-苹果酸酶基因家族及启动子克隆分析. 西北植物学报, 2022, 42(5): 760-769. | |
| 21 | Wu X F, Yang F, Yan X Y, et al. Response of hydraulic resistances of soil-Robinia pseudoacacia system of water stress. Research of Soil and Water Conservation, 2022, 29(2): 274-280. |
| 武小飞, 杨帆, 岩晓莹, 等. 土壤-刺槐系统水流阻力对水分胁迫的响应. 水土保持研究, 2022, 29(2): 274-280. | |
| 22 | Li L, Li N H, Jiang S M. Experimental guide of plant physiology module. Beijing: Science Press, 2009: 37-103. |
| 李玲, 李娘辉, 蒋素梅. 植物生理学模块实验指导. 北京: 科学出版社, 2009: 37-103. | |
| 23 | Li L, Yu D, Zhao F, et al. Genome-wide analysis of the calcium-dependent protein kinase gene family in Gossypium raimondii. Journal of Integrative Agriculture, 2015, 14(1): 29-41. |
| 24 | Pandey G K, Kanwar P, Singh A, et al. Calcineurin B-like protein-interacting protein kinase CIPK21 regulates osmotic and salt stress responses in Arabidopsis. Plant Physiology, 2015, 169(1): 780-792. |
| 25 | Ma Q J, Sun M H, Lu J, et al. An apple sucrose transporter MdSUT2.2 is a phosphorylation target for protein kinase MdCIPK22 in response to drought. Plant Biotechnology Journal, 2019, 17(3): 625-637. |
| 26 | Zhang H, Liu D, Yang B, et al. Arabidopsis CPK6 positively regulates ABA signaling and drought tolerance through phosphorylating ABA-responsive element-binding factors. Journal of Experimental Botany, 2020, 71(1): 188-203. |
| 27 | Ma X. Responses and regulation mechanisms of CaCIPKs under drought and cold stresses in Capsicum annuum. Xianyang: Northwest A & F University, 2022. |
| 马潇. 辣椒CaCIPKs对干旱和低温胁迫的响应及其调控机理研究. 咸阳: 西北农林科技大学, 2022. | |
| 28 | Liu D D, Zhu M, Hao L L, et al. GhMAPKKK49, a novel cotton (Gossypium hirsutum L.) MAPKKK gene, is involved in diverse stress responses. Acta Physiologiae Plantarum, 2015, 38(1): 1-12. |
| 29 | Zhu X, Zhang N, Liu X, et al. Mitogen-activated protein kinase 11(MAPK11) maintains growth and photosynthesis of potato plant under drought condition. Plant Cell Reports, 2021, 40(3): 491-506. |
| 30 | Mahmood T, Khalid S, Abdullah M, et al. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells, 2019, 9(1): 105. |
| 31 | Qi W L, Ren Y H, Yang C R, et al. Signal transduction and transcriptome analysis of reactive oxygen species in mulberry under drought stress. Agricultural Research in the Arid Areas, 2023, 41(2): 50-60. |
| 祁伟亮, 任迎虹, 杨财容, 等. 干旱胁迫下桑树活性氧信号传导及转录组分析. 干旱地区农业研究, 2023, 41(2): 50-60. | |
| 32 | Gao P F, Zhang J, Fan W F, et al. Effects of drought stress on root characteristics structure and physiological characteristics of Potentilla bifurca var. glabrata. Acta Prataculturae Sinica, 2022, 31(2): 203-212. |
| 高鹏飞, 张静, 范卫芳, 等. 干旱胁迫对光叉委陵菜根系特征、结构和生理特性的影响. 草业学报, 2022, 31(2): 203-212. | |
| 33 | Guan S J, Wang N, Xu R R, et al. Photosynthesis, antioxidant enzyme activity, and transcriptome sequencing analyses of Glycyrrhiza uralensis seedlings in response to drought stress. Pratacultural Science, 2021, 38(11): 2176-2190. |
| 关思静, 王楠, 徐蓉蓉, 等. 甘草幼苗响应干旱胁迫的光合、抗氧化特性及转录组分析. 草业科学, 2021, 38(11): 2176-2190. | |
| 34 | Wan L Y, Su W, Li B, et al. Molecular analysis of formation of drought tolerance traits in peanut. Chinese Journal of Oil Crop Sciences, 2018, 40(3): 335-343. |
| 万丽云, 苏威, 李蓓, 等. 花生苗期干旱处理后转录和代谢通路分析. 中国油料作物学报, 2018, 40(3): 335-343. | |
| 35 | Zsigmond L, Rigó G, Szarka A, et al. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiology, 2008, 146(4): 1721-1737. |
| 36 | Su P X, An L Z, Ma R J, et al. Kranz anatomy and C4 photosynthetic characteristics of two desert pants, Haloxylon ammodendron and Calligonum mongolicum. Acta Phytoecologica Sinica, 2005, 29(1): 1-7. |
| 苏培玺, 安黎哲, 马瑞君, 等. 荒漠植物梭梭和沙拐枣的花环结构及C4光合特征. 植物生态学报, 2005, 29(1): 1-7. | |
| 37 | Han R L, Li L X, Liang Z S. Seabuckthorn relative membrane conductivity and osmotic adjustment under drought stress. Acta Botanica Boreali-Occidentalia Sinica, 2003, 23(1): 23-27. |
| 韩蕊莲, 李丽霞, 梁宗锁. 干旱胁迫下沙棘叶片细胞膜透性与渗透调节物质研究. 西北植物学报, 2003, 23(1): 23-27. |
| [1] | 张鑫苗, 伍国强, 魏明. MAPK在植物响应逆境胁迫中的作用[J]. 草业学报, 2024, 33(1): 182-197. |
| [2] | 姜瑛, 张辉红, 魏畅, 徐正阳, 赵颖, 刘芳, 李鸽子, 张雪海, 柳海涛. 外源褪黑素对干旱胁迫下玉米幼苗根系发育及生理生化特性的影响[J]. 草业学报, 2023, 32(9): 143-159. |
| [3] | 王宝强, 马文静, 王贤, 朱晓林, 赵颖, 魏小红. 一氧化氮对干旱胁迫下紫花苜蓿幼苗次生代谢产物的影响[J]. 草业学报, 2023, 32(8): 141-151. |
| [4] | 史先飞, 高宇, 黄旭升, 周雅莉, 蔡桂萍, 李昕儒, 李润植, 薛金爱. 油莎豆CeWRKY转录因子响应非生物胁迫的功能表征[J]. 草业学报, 2023, 32(8): 186-201. |
| [5] | 孙守江, 毛培胜, 豆丽茹, 贾志程, 孙铭, 马馼, 欧成明, 王娟. 活性氧及染色体端粒调控种子老化研究[J]. 草业学报, 2023, 32(8): 202-213. |
| [6] | 张一龙, 李雯, 喻启坤, 李培英, 孙宗玖. 狗牙根叶与根氮代谢对不同干旱胁迫的响应机制[J]. 草业学报, 2023, 32(7): 175-187. |
| [7] | 张浩, 胡海英, 李惠霞, 贺海明, 马霜, 马风华, 宋柯辰. 荒漠草原优势植物牛枝子对干旱胁迫的生理响应与转录组分析[J]. 草业学报, 2023, 32(7): 188-205. |
| [8] | 梁佳, 胡朝阳, 谢志明, 马刘峰, 陈芸, 方志刚. 外源褪黑素缓解甜高粱幼苗干旱胁迫的生理效应[J]. 草业学报, 2023, 32(7): 206-215. |
| [9] | 冯华昊, 王涵, 周建祯, 张晗, 唐韬, 彭燕. 白三叶耐铝种质筛选及耐铝评价指标分析[J]. 草业学报, 2023, 32(6): 100-111. |
| [10] | 陈晓明, 韩东英, 宋桂龙. 砷(As)胁迫对海滨雀稗As吸收特征及根系形态影响[J]. 草业学报, 2023, 32(6): 112-119. |
| [11] | 崔婷, 王勇, 马晖玲. 外源IAA作用下草地早熟禾中调控Cd长距离运输的关键基因表达及其代谢通路分析[J]. 草业学报, 2023, 32(6): 146-156. |
| [12] | 张适阳, 刘凤民, 崔均涛, 何磊, 冯月燕, 张伟丽. 三种外源物质对低温胁迫下柱花草生理与荧光特性的影响[J]. 草业学报, 2023, 32(6): 85-99. |
| [13] | 李艳鹏, 魏娜, 翟庆妍, 李杭, 张吉宇, 刘文献. 全基因组水平白花草木樨TCP基因家族的鉴定及在干旱胁迫下表达模式分析[J]. 草业学报, 2023, 32(4): 101-111. |
| [14] | 尚盼盼, 曾兵, 屈明好, 李明阳, 杨兴云, 郑玉倩, 沈秉娜, 毕磊, 杨成, 曾兵. 红三叶响应淹水胁迫的相关通路及差异表达基因分析[J]. 草业学报, 2023, 32(4): 112-128. |
| [15] | 田政, 杨正禹, 陆忠杰, 罗奔, 张茂, 董瑞. 44个紫花苜蓿品种的酸铝适应性与耐受性评价[J]. 草业学报, 2023, 32(3): 142-151. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||