

ISSN 1004-5759 CN 62-1105/S


草业学报 ›› 2026, Vol. 35 ›› Issue (2): 208-220.DOI: 10.11686/cyxb2025112
收稿日期:2025-03-21
修回日期:2025-05-06
出版日期:2026-02-20
发布日期:2025-12-24
通讯作者:
李州
作者简介:Corresponding author. E-mail: lizhou1986814@163.com基金资助:
Yi-lin DI( ), Si-tian LIU, Xin-ying LIU, Yong DU, Zhou LI(
), Si-tian LIU, Xin-ying LIU, Yong DU, Zhou LI( )
)
Received:2025-03-21
Revised:2025-05-06
Online:2026-02-20
Published:2025-12-24
Contact:
Zhou LI
摘要:
镉(Cd)是土壤中常见的重金属污染物,严重降低了草坪草的生长发育。γ-氨基丁酸(GABA)是植物体内重要的生长调节物质,广泛参与调节植物非生物胁迫耐受性。本研究探讨了外施GABA对Cd胁迫下匍匐翦股颖叶片内源GABA含量、叶绿素荧光性能、细胞膜稳定性及不同部位Cd吸收和转运的影响。研究结果显示,Cd胁迫显著降低了匍匐翦股颖叶片相对含水量、叶绿素含量和光化学效率,导致膜脂过氧化伤害和细胞膜稳定性降低。外源添加 0.5 mmol·L-1 GABA能显著提高Cd胁迫下叶片内源GABA积累、叶绿素含量和相对含水量,抑制丙二醛产生和电解质渗透率上升,并提高光化学效率(Fv/Fm)和叶片健康指数(PIABS),有效缓解了Cd胁迫对细胞造成的伤害。此外,Cd胁迫下匍匐翦股颖叶片和根系中Cd含量急剧升高,但Cd在根系中的富集量远远大于地上部分。外施GABA能显著降低叶片中Cd的积累,但提高了Cd在根系中的积累,表明GABA抑制了Cd从根系向地上部分的转运。这一过程可能与GABA显著上调了根系中AsZIP2、AsNRAMP1和AsNRAMP5的表达量以及下调了叶片中AsNRAMP1和AsNRAMP5的表达量有关。GABA也显著上调了Cd胁迫下根系中AsHMA1、AsHMA3、AsABCC2和AsABCC4的表达量,有助于提高根系Cd离子区隔化到液泡中,降低根系Cd的毒性。这些研究结果不仅丰富了GABA在调节植物耐Cd性上的作用机理,也为冷季型草坪草耐Cd栽培管理提供了技术参考。
第乙林, 刘思甜, 刘欣影, 杜泳, 李州. γ-氨基丁酸对镉胁迫下匍匐翦股颖耐受性及镉吸收转运的影响[J]. 草业学报, 2026, 35(2): 208-220.
Yi-lin DI, Si-tian LIU, Xin-ying LIU, Yong DU, Zhou LI. Effects of γ-aminobutyric acid on cadmium stress tolerance and cadmium uptake and transport in creeping bentgrass[J]. Acta Prataculturae Sinica, 2026, 35(2): 208-220.
基因 Gene | 上游引物 Forward primer (5'-3') | 下游引物 Reverse primer (5'-3') | 退火温度 Annealing temperature (Tm, ℃) |
|---|---|---|---|
| Asβ-actin | CCTTTTCCAGCCATCTTTCA | GAGGTCCTTCCTGATATCCA | 54 |
| AsHMA1 | GGCTTGTCAGTCTATTGCTTT | GCTTCACTTTCACAGTTTGGT | 54 |
| AsHMA3 | CTGGGAGACGGGAACAGAG | CAGCAGTGGCAGGCTTTATC | 58 |
| AsZIP2 | ATCACTCCACGGCATCAAT | CTTTCGTTTCAGCGACTCC | 54 |
| AsABCC2 | AGAGCTGTTTATTCCGATTCA | CTATTTGCCCCTGAGGTATG | 54 |
| AsABCC4 | AAAGGAGAGCGGACGAGTAA | GAAGCGTAGACACCAAGGAAC | 56 |
| AsNRAMP1 | ACTCTTCAATCCGCACCTCT | TTCCTCACCCAGTTCTTCATC | 56 |
| AsNRAMP5 | AGCAGCAGAAGCAAGATGG | CAGAGGGAAGACGACGATG | 56 |
表1 实时荧光定量PCR引物
Table 1 Real-time PCR primers
基因 Gene | 上游引物 Forward primer (5'-3') | 下游引物 Reverse primer (5'-3') | 退火温度 Annealing temperature (Tm, ℃) |
|---|---|---|---|
| Asβ-actin | CCTTTTCCAGCCATCTTTCA | GAGGTCCTTCCTGATATCCA | 54 |
| AsHMA1 | GGCTTGTCAGTCTATTGCTTT | GCTTCACTTTCACAGTTTGGT | 54 |
| AsHMA3 | CTGGGAGACGGGAACAGAG | CAGCAGTGGCAGGCTTTATC | 58 |
| AsZIP2 | ATCACTCCACGGCATCAAT | CTTTCGTTTCAGCGACTCC | 54 |
| AsABCC2 | AGAGCTGTTTATTCCGATTCA | CTATTTGCCCCTGAGGTATG | 54 |
| AsABCC4 | AAAGGAGAGCGGACGAGTAA | GAAGCGTAGACACCAAGGAAC | 56 |
| AsNRAMP1 | ACTCTTCAATCCGCACCTCT | TTCCTCACCCAGTTCTTCATC | 56 |
| AsNRAMP5 | AGCAGCAGAAGCAAGATGG | CAGAGGGAAGACGACGATG | 56 |

图1 外源γ-氨基丁酸对CdCl2胁迫下匍匐翦股颖生长和叶片叶绿素含量的影响不同字母表示在0.05水平上差异显著(P<0.05)。下同。Different letters indicated significant difference at P<0.05 level. The same below.
Fig.1 Effects of exogenous γ-aminobutyric acid on growth and chlorophyll content in A. stolonifera under CdCl2 stress

图3 外源γ-氨基丁酸对CdCl2胁迫下匍匐翦股颖膜透性、相对含水量及γ-氨基丁酸合成的影响
Fig.3 Effects of exogenous γ-aminobutyric acid on membrane permeability, relative water content and GABA synthesis of A. stolonifera under CdCl2 stress
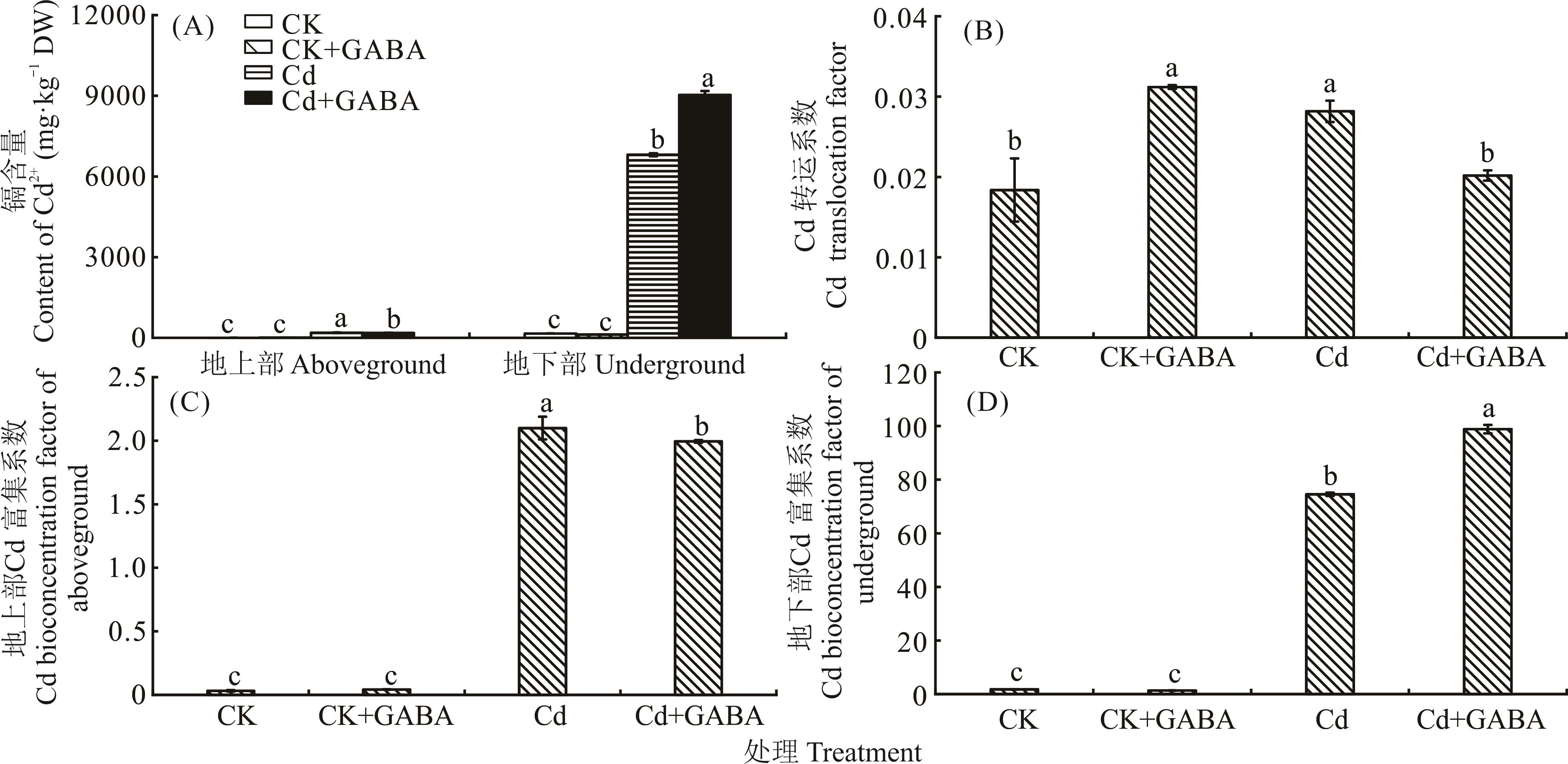
图4 外源γ-氨基丁酸对CdCl2胁迫下匍匐翦股颖Cd含量及离子分配的影响
Fig.4 Effect of exogenous γ-aminobutyric acid on Cd2+ content and ion partitioning in A. stolonifera under CdCl2 stress
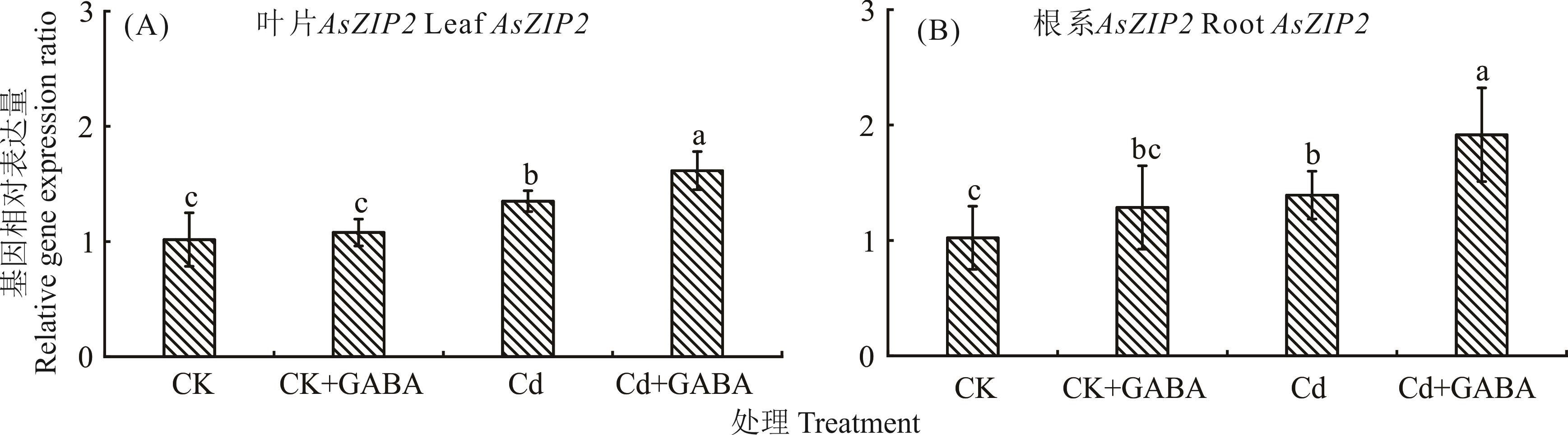
图5 外源γ-氨基丁酸对CdCl2胁迫下匍匐翦股颖 ZIP 基因家族表达的影响
Fig.5 Effect of exogenous γ-aminobutyric acid on the expression of ZIP gene family in A. stolonifera under CdCl2 stress

图6 外源γ-氨基丁酸对CdCl2 胁迫下匍匐翦股颖 NRAMP 基因家族表达的影响
Fig.6 Effect of exogenous γ-aminobutyric acid on the expression of NRAMP gene family in A. stolonifera under CdCl2 stress

图7 外源γ-氨基丁酸对CdCl2 胁迫下匍匐翦股颖 HMA 基因家族表达的影响
Fig.7 Effect of exogenous γ-aminobutyric acid on the expression of HMA gene family in A. stolonifera under CdCl2 stress

图8 外源γ-氨基丁酸对CdCl2 胁迫下匍匐翦股颖 ABCC 基因家族表达的影响
Fig.8 Effect of exogenous γ-aminobutyric acid on the expression of ABCC gene family in A. stolonifera under CdCl2 stress
| [1] | Dradrach A, Karczewska A, Bogacz A, et al. Accumulation of potentially toxic metals in ryegrass (Lolium perenne L.) and other components of lawn vegetation in variously contaminated sites of urban areas. Sustainability, 2024, 16(18): 8040. |
| [2] | Gao L, Wang S F, Zou D C, et al. Physiological responses of low- and high-cadmium accumulating Robinia pseudoacacia-rhizobium symbioses to cadmium stress. Environmental Pollution, 2024, 345(6): 123456. |
| [3] | Anjum S A, Tanveer M, Hussain S, et al. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean-Soil, Air, Water, 2016, 44(1): 29-36. |
| [4] | Guo J J, Qin S Y, Rengel Z, et al. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicology and Environmental Safety, 2019, 172(6): 380-387. |
| [5] | Lin J N, Lin L, Shi J A, et al. Growth and metabolic differences in the potential of phytoremediation between two hybrid bermudagrasses in roots, stems, and leaves under cadmium stress. Environmental and Experimental Botany, 2024, 222(6): 105767. |
| [6] | Liu P, Sun L, Zhang Y, et al. The metal tolerance protein OsMTP11 facilitates cadmium sequestration in the vacuoles of leaf vascular cells for restricting its translocation into rice grains. Molecular Plant, 2024, 17(11): 1733-1752. |
| [7] | Wu X, Chen J H, Yue X M, et al. The zinc-regulated protein (ZIP) family genes and glutathione s-transferase (GST) family genes play roles in Cd resistance and accumulation of pak choi (Brassica campestris ssp. chinensis). Ecotoxicology and Environmental Safety, 2019, 183(17): 109571. |
| [8] | Zheng X, Chen L, Li X F. Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Botanical Studies, 2018, 59(22): 1-10. |
| [9] | Ishikawa S, Ishimaru Y, Igura M, et al. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(47): 19166-19171. |
| [10] | Takahashi R, Ishimaru Y, Senoura T, et al. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. Journal of Experimental Botany, 2011, 62(14): 4843-4850. |
| [11] | Miyadate H, Adachi S, Hiraizumi A, et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytologist, 2011, 189(1): 190-199. |
| [12] | Brunetti P, Zanella L, De Paolis A, et al. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. Journal of Experimental Botany, 2015, 66(13): 3815-3829. |
| [13] | Faizan M, Alam P, Hussain A, et al. Phytochelatins: A key regulator against heavy metal toxicity in plants. Plant Stress, 2024, 11(1): 100355. |
| [14] | Song W Y, Park J, Mendoza-Cózatl D G, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(49): 21187-21192. |
| [15] | Ramesh S A, Tyerman S D, Gilliham M, et al. γ-aminobutyric acid (GABA) signalling in plants. Cellular and Molecular Life Sciences, 2017, 74(22): 1577-1603. |
| [16] | Deewatthanawong R, Rowell P, Watkins C B, et al. γ-aminobutyric acid (GABA) metabolism in CO2 treated tomatoes. Postharvest Biology and Technology, 2010, 57(2): 97-105. |
| [17] | Dolphen R, Thiravetyan P. Exogenous γ-aminobutyric acid and Bacillus pumilus reduce arsenic uptake and toxicity in rice. Environmental Science and Pollution Research, 2024, 31(7): 10609-10620. |
| [18] | Kalhor M S, Aliniaeifard S, Seif M, et al. Enhanced salt tolerance and photosynthetic performance: Implication of γ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiology and Biochemistry, 2018, 130(9): 157-172. |
| [19] | Zhu G X, Xiao H Y, Guo Q J, et al. Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicology and Environmental Safety, 2018, 158(12): 300-308. |
| [20] | Waris Z, Noreen Z, Shah A A, et al. Efficacy of γ-aminobutyric acid (GABA) on physio-biochemical attributes of lettuce (Lactuca sativa L.) under cadmium toxicity. Journal of Plant Growth Regulation, 2023, 42(8): 5041-5057. |
| [21] | He G Q, Zhang H B, Liu S Q, et al. Exogenous γ-glutamic acid (GABA) induces proline and glutathione synthesis in alleviating Cd-induced photosynthetic inhibition and oxidative damage in tobacco leaves. Journal of Plant Interactions, 2021, 16(1): 296-306. |
| [22] | Seifikalhor M, Aliniaeifard S, Bernard F, et al. γ-aminobutyric acid confers cadmium tolerance in maize plants by concerted regulation of polyamine metabolism and antioxidant defense systems. Scientific Reports, 2020, 10(1): 3356. |
| [23] | Ashraf U, Anjum S A, Rasul F, et al. GABA-mediated tolerance in fragrant rice to individual and interactive Pb and Cd stress. Research Square, 2024, 7(1): 30-45. |
| [24] | Zhao Y T, Song X T, Zhong D B, et al. γ-aminobutyric acid (GABA) regulates lipid production and cadmium uptake by Monoraphidium sp. QLY-1 under cadmium stress. Bioresource Technology, 2020, 297(3): 122500. |
| [25] | Li Y X, Li Y H, Cui Y L, et al. GABA-mediated inhibition of cadmium uptake and accumulation in apples. Environmental Pollution, 2022, 300(9): 118867. |
| [26] | Li W Z, Li X F, Zhou K X, et al. Exogenous γ-aminobutyric acid (GABA) improves the cadmium phytoremediation capacity of Solanum nigrum var. humile under cadmium stress. Environmental Progress & Sustainable Energy, 2024, 43(4): e14364. |
| [27] | Ji C D, Zhang D G, Zhu J, et al. Effects of high temperature stress on some physiological characteristics and regeneration ability of Agrostis stolonifera green turfgrass. Chinese Agricultural Science Bulletin, 2007, 23(1): 221-224. |
| 姬承东, 张德罡, 朱钧, 等. 高温对匍匐翦股颖果岭草坪草生理特性及再生性的影响. 中国农学通报, 2007, 23(1): 221-224. | |
| [28] | Wang H H. Effect of calcium salt on salt tolerance of creeping bentgrass under NaCl stress. Lanzhou: Lanzhou University, 2021. |
| 王慧慧. 钙盐对 NaCl 胁迫下匍匐翦股颖耐盐性影响. 兰州: 兰州大学, 2021. | |
| [29] | Li Z, Zhou M, Qi H Y, et al. Foliar application of diethyl aminoethyl hexanoate (DA-6) alleviated summer bentgrass decline and heat damage to creeping bentgrass. Crop Science, 2024, 64(2): 1039-1050. |
| [30] | Arnon D. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiology, 1949, 24(1): 1-15. |
| [31] | Barr H D, Weatherley P E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Australian Journal of Biological Sciences, 1962, 15(3): 413-428. |
| [32] | Blum A, Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Science, 1981, 21(1): 43-47. |
| [33] | Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 2001, 25(4): 402-408. |
| [34] | Wu Z C, Zhao X H, Sun X C, et al. Antioxidant enzyme systems and the ascorbate-glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere, 2015, 138(21): 526-536. |
| [35] | Deng G, Li M, Li H, et al. Exposure to cadmium causes declines in growth and photosynthesis in the endangered aquatic fern (Ceratopteris pteridoides). Aquatic Botany, 2014, 112(1): 23-32. |
| [36] | Santos L R, Batista B L, Lobato A K S. Brassinosteroids mitigate cadmium toxicity in cowpea plants. Photosynthetica, 2018, 56(2): 591-605. |
| [37] | Zhang H H, Xu Z S, Guo K W, et al. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicology and Environmental Safety, 2020, 202(16): 110856. |
| [38] | Benavides M P, Gallego S M, Tomaro M L. Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 2005, 17(1): 21-34. |
| [39] | Wodala B, Eitel G, Gyula T N, et al. Monitoring moderate Cu and Cd toxicity by chlorophyll fluorescence and P700 absorbance in pea leaves. Photosynthetica, 2012, 50(2): 380-386. |
| [40] | Dhir B, Sharmila P, Saradhi P P. Photosynthetic performance of Salvinia natans exposed to chromium and zinc rich wastewater. Brazilian Journal of Plant Physiology, 2008, 20(1): 61-70. |
| [41] | Feng Y Z, Zhao Y, Wang B P, et al. Effects of drought rehydration on photosynthesis and chlorophyll fluorescence of Paulownia catalpifolia seedlings. Journal of Central South University of Forestry & Technology, 2020, 40(4): 1-8. |
| 冯延芝, 赵阳, 王保平, 等. 干旱复水对楸叶泡桐幼苗光合和叶绿素荧光的影响. 中南林业科技大学学报, 2020, 40(4): 1-8. | |
| [42] | Xiang W, Cheng X R, Xu H D, et al. Effects of light and N∶P ratio on photosynthetic characteristics of three typical tree species. Forest Research, 2023, 36(1): 179-190. |
| 向旺, 成向荣, 徐海东, 等. 光照和氮磷供应比对 3 种典型乔木幼苗光合生理特性的影响. 林业科学研究, 2023, 36(1): 179-190. | |
| [43] | Jin C, Zha T S, Jia X, et al. Dynamics of chlorophyll fluorescence parameters under drought condition for three desert shrub species. Journal of Beijing Forestry University, 2020, 42(8): 72-80. |
| 靳川, 查天山, 贾昕, 等. 干旱环境 3 种荒漠灌木叶绿素荧光参数动态. 北京林业大学学报, 2020, 42(8): 72-80. | |
| [44] | Yang H Z, Yuan Y, Liu X Y, et al. Phytohormonal homeostasis, chloroplast stability, and heat shock transcription pathways related to the adaptability of creeping bentgrass species to heat stress. Protoplasma, 2025, 262(1): 649-665. |
| [45] | Li D, Li L, Xiao G N, et al. Effects of elevated CO2 on energy metabolism and γ-aminobutyric acid shunt pathway in postharvest strawberry fruit. Food Chemistry, 2018, 265(28): 281-289. |
| [46] | Qin B, Sun M L, Liu H Z, et al. Alfalfa MsGAD2 induces γ-aminobutyric acid accumulation and enhances Cd resistance in transgenic tobacco. Environmental and Experimental Botany, 2025, 229(1): 106058. |
| [47] | Lin L, Lin J N, Zhou M, et al. Lipid remodelling and the conversion of lipids into sugars associated with tolerance to cadmium toxicity during white clover seed germination. Physiologia Plantarum, 2024, 176(4): e14433. |
| [48] | Lv Y Y, Zhao Y T S, He Y, et al. Synergistic effects of γ-aminobutyric acid and melatonin on seed germination and cadmium tolerance in tomato. Plant Signaling & Behavior, 2023, 18(1): 2216001. |
| [49] | Zhao F J, Tang Z, Song J J, et al. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Molecular Plant, 2022, 15(1): 27-44. |
| [50] | Cuypers A, Plusquin M, Remans T, et al. Cadmium stress: An oxidative challenge. Biometals, 2010, 23(2): 927-940. |
| [51] | Hao X H, Liu K X, Zhang M Y. Effect of exogenous γ-aminobutyric acid on physiological property, antioxidant activity, and cadmium uptake of quinoa seedlings under cadmium stress. Bioscience Reports, 2024, 44(6): BSR20240215. |
| [52] | Gallego S M, Pena L B, Barcia R A, et al. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environmental and Experimental Botany, 2012, 83(9): 33-46. |
| [53] | Cao Y Q, Nie Q K, Gao Y, et al. The studies on cadmium and its chelate related transporters in plants. Crops, 2018, 3(3): 15-24. |
| [54] | Milner M J, Seamon J, Craft E, et al. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. Journal of Experimental Botany, 2013, 64(1): 369-381. |
| [55] | Kanwal F, Riaz A, Ali S, et al. NRAMPs and manganese: Magic keys to reduce cadmium toxicity and accumulation in plants. Science of the Total Environment, 2024, 921(16): 171005. |
| [56] | Zhang J, Zhang M, Song H Y, et al. A novel plasma membrane-based NRAMP transporter contributes to Cd and Zn hyperaccumulation in Sedum alfredii Hance. Environmental and Experimental Botany, 2020, 176(8): 104121. |
| [57] | Zhang W W, Yue S Q, Song J F, et al. MhNRAMP1 from Malus hupehensis exacerbates cell death by accelerating cd uptake in tobacco and apple calli. Frontiers in Plant Science, 2020, 11(7): 957. |
| [58] | Di Y L, Cao Y Q, Peng D D, et al. AsGAD1 cloned from creeping bentgrass modulates cadmium tolerance of Arabidopsis thaliana by remodelling membrane lipids and cadmium uptake, transport and chelation. Physiologia Plantarum, 2025, 177(1): e70063. |
| [59] | Khan N, You F M, Datla R, et al. Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.). BMC Genomics, 2020, 21(7): 1-14. |
| [60] | Argüello J M, Eren E, González-Guerrero M. The structure and function of heavy metal transport P 1B-ATPases. Biometals, 2007, 20(1): 233-248. |
| [61] | Mikkelsen M D, Pedas P, Schiller M, et al. Barley HvHMA1 is a heavy metal pump involved in mobilizing organellar Zn and Cu and plays a role in metal loading into grains. PLoS One, 2012, 7(11): e49027. |
| [62] | Ueno D, Milner M J, Yamaji N, et al. Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. The Plant Journal, 2011, 66(5): 852-862. |
| [63] | Park J, Song W Y, Ko D, et al. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. The Plant Journal, 2012, 69(2): 278-288. |
| [1] | 宋淑珍, 朱才业, 刘立山, 宫旭胤, 雒瑞瑞. 断尾对兰州大尾羊脂肪细胞结构和脂肪代谢相关基因表达的影响[J]. 草业学报, 2024, 33(7): 94-104. |
| [2] | 李强, 康璠, 薛晴, 陈斌, 孙颖. 神农香菊R2R3-MYB转录因子CiMYB4在镉胁迫中的功能分析[J]. 草业学报, 2024, 33(5): 128-142. |
| [3] | 孔海明, 宋家兴, 杨静, 李倩, 杨培志, 曹玉曼. 紫花苜蓿CAMTA基因家族鉴定及其在非生物胁迫下的表达模式分析[J]. 草业学报, 2024, 33(5): 143-154. |
| [4] | 孟超楠, 赵玉洁, 陈佳欣, 张旖璐, 王彦佳, 冯丽荣, 孙玉刚, 郭长虹. 2株青贮玉米根际固氮菌的筛选鉴定及促生作用研究[J]. 草业学报, 2024, 33(3): 174-185. |
| [5] | 陈嘉慧, 刘文献. 重要牧草组学数据图形可视化展示工具的构建及应用[J]. 草业学报, 2024, 33(2): 57-67. |
| [6] | 管瑾, 郭一荻, 刘凌云, 尹淑霞, 滕珂. 结缕草叶肉细胞原生质体瞬时基因表达系统的构建[J]. 草业学报, 2023, 32(7): 61-71. |
| [7] | 刘牧野, 郭丽珠, 岳跃森, 武菊英, 范希峰, 肖国增, 滕珂. 干旱胁迫下不同性别野牛草生理及抗氧化酶基因表达差异[J]. 草业学报, 2023, 32(10): 93-103. |
| [8] | 许浩宇, 赵颖, 阮倩, 朱晓林, 王宝强, 魏小红. 不同混合盐碱下藜麦幼苗的抗性研究[J]. 草业学报, 2023, 32(1): 122-130. |
| [9] | 曾令霜, 李培英, 孙宗玖, 孙晓梵. 两类新疆狗牙根抗旱基因型抗氧化酶保护系统及其基因表达差异分析[J]. 草业学报, 2022, 31(7): 122-132. |
| [10] | 张晴, 邢静, 姚佳明, 殷庭超, 黄心如, 何悦, 张敬, 徐彬. 多年生黑麦草细胞分裂素信号通路B类ARR转录因子LpARR10的耐镉功能分析[J]. 草业学报, 2022, 31(5): 135-143. |
| [11] | 赵利清, 郝志刚, 崔笑岩, 彭向永. 赤霉素及其抑制剂调控草地早熟禾生长及赤霉素相关基因表达的研究[J]. 草业学报, 2022, 31(3): 85-91. |
| [12] | 张国香, 郭卫冷, 毕铭钰, 张力爽, 王丹, 郭长虹. 紫花苜蓿CAX基因家族鉴定及其对非生物胁迫的响应分析[J]. 草业学报, 2022, 31(12): 106-117. |
| [13] | 赵宁, 马晖玲, 张然, 张金青, 史毅. 丁二醇对热胁迫下匍匐翦股颖内源激素及其相关基因表达水平的调控[J]. 草业学报, 2022, 31(12): 118-132. |
| [14] | 魏娜, 李艳鹏, 马艺桐, 刘文献. 全基因组水平紫花苜蓿TCP基因家族的鉴定及其在干旱胁迫下表达模式分析[J]. 草业学报, 2022, 31(1): 118-130. |
| [15] | 马倩, 闫启, 张正社, 吴凡, 张吉宇. 紫花苜蓿CCoAOMT基因家族的鉴定、进化及表达分析[J]. 草业学报, 2021, 30(11): 144-156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||